Functional capacity impairment is a crucial consequence of chronic obstructive pulmonary disease (COPD). Although it can be identified with simple tests, such as the sit-to-stand tests, its prevalence, relation with disease severity, and the characteristics of people presenting this impairment remain unknown.
ObjectiveTo explore the functional capacity of people with COPD.
MethodsA cross-sectional study with people with COPD and age-/sex-matched healthy controls was conducted. Functional capacity was assessed with the 5-repetitions (5-STS) and the 1-minute (1-minSTS) sit-to-stand tests. People with COPD were grouped according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classifications. Comparisons between people with COPD and healthy controls, and among GOLD groups were established. Associations between symptoms, muscle strength, quality of life, and measures of functional capacity were explored.
Results302 people with COPD [79% male; mean (SD) 68 (10) years old] and 304 healthy controls [75% male; 66 (9) years old] were included. 23% of people with COPD presented impairment in the 5-STS and 33% in the 1-minSTS. People with COPD from all GOLD classifications presented significantly lower functional capacity than healthy controls (5-STS: COPD median [1st quartile; 3rd quartile] 8.4 [6.7; 10.6] versus healthy 7.4 [6.2; 9.3] s; 1-minSTS: COPD 27 [21; 35] vs healthy 35 [29; 43] reps). Correlations with symptoms, muscle strength, and quality of life were mostly weak (5-STS: rs [-0.34; 0.33]; 1-minSTS: rs [-0.47; 0.40]).
ConclusionPeople with COPD have decreased functional capacity independently of their GOLD classifications. The prevalence of functional impairment is 23–33%. Because impaired functional capacity is a treatable trait not accurately reflected by other outcomes, comprehensive assessment and management is needed.
Chronic obstructive pulmonary disease (COPD) leads to a variety of pulmonary and non-pulmonary manifestations, including decreased functional status, which is the main reason why patients seek medical help.1-4 Impaired functional status further contributes to health status decline and is a predictor of exacerbations, hospitalizations, and mortality.3,5,6 Nevertheless, functional status impairment and its relationship with disease severity are still not fully understood.7-9 Thus, research on functional status and its assessment and treatment in people with COPD is a recognized priority.1,3,6,7,10
Functional status refers to people's ability to satisfy life's necessities, i.e., the activities they do in the normal course of their lives to meet basic needs, fulfill usual roles, and maintain their health and well-being.11 It comprises four dimensions: capacity, performance, reserve, and capacity utilization.11 Among these, functional capacity (FC), i.e., individual's maximum potential to perform activities people do in the normal course of their lives,11 has been recognized as a key outcome to be assessed in people with COPD.12 Although several measures have been proposed to assess the FC of people with COPD, most studies have used the 6-minute walk test (6MWT),3,6,13 which requires space, time, and trained staff.13 To overcome these barriers, simpler and quicker tests, such as the sit-to-stand tests, which need limited space and equipment, and are therefore feasible across different settings, have emerged.1,14,15 Among the sit-to-stand tests, the 5-repetitions sit-to-stand (5-STS) and the 1-minute sit-to-stand (1-minSTS) versions have been the most used in people with COPD due to their relationship with the 6MWT and ability to predict exacerbations and mortality.15-23 To date, the prevalence of FC impairment (assessed by the 5-STS and 1-minSTS), its distribution across disease severity, and the characteristics of people presenting such impairment remain unknown. We believe this knowledge would improve our understanding of a highly meaningful health domain for the daily life of people with COPD and could guide tailored interventions.
This study aimed to explore the FC of people with COPD using sit-to-stand tests. Specifically, we compared the FC of people with COPD with matched healthy individuals; explored the prevalence of FC impairment in people with COPD and its distribution across disease stages and groups; the characteristics of those with FC impairment; the correlation between clinical and patient-reported outcomes and measures of FC; and the predictive ability of clinical and patient-reported outcomes to identify FC impairment in people with COPD.
MethodsStudy design and ethicsThis was a cross-sectional multicenter study, focused on clinical data, embedded in the observational longitudinal study GENIAL (PTDC/DTP-PIC/2284/2014) which aimed to establish the role of genetic mutations on the development and trajectory of COPD and identify clinical markers to detect exacerbations. The cross-sectional part was conducted between September 2016 and June 2019 in people with COPD and age-/sex-matched healthy controls. A sample size calculation was performed for GENIAL to find a statistical difference between groups (i.e., COPD vs healthy) in one genetic variation of 10%, with the largest standard deviation (power 80%, α=0.05). It was estimated that a sample of 392 individuals in each group would be needed. Approval for this study was obtained from the ethical committees of the University of Aveiro (13APR’2016:8/2015), Administração Regional de Saúde do Centro (3NOV’2016:64/2016), Centro Hospitalar do Baixo Vouga (22MAR’2017:777,638), Hospital Pedro Hispano (17FEB’2017:10/CE/JAS), Hospital Distrital da Figueira da Foz (18JUL’2017), Hospital da Misericórdia da Mealhada (9NOV’2018), and the National Data Protection Committee (8828/2016). All participants provided written informed consent before any data collection. This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.24
ParticipantsPeople diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria,2 who were clinically stable for 1 month (i.e., no hospitalizations, exacerbations, or changes in respiratory medication), were recruited from 4 hospitals and 8 primary healthcare centers in the North and Central regions of Portugal. Healthy adults presenting no chronic respiratory diseases were recruited from the community (e.g., day centers, civil parishes) in the same regions. Exclusion criteria for both populations included: i) history of an acute cardiac or respiratory condition in the previous month; ii) presence of cardiac or neuromusculoskeletal diseases that impaired the ability to perform the assessments; iii) signs of cognitive impairment, and iv) history of neoplasia or immunological disease. In both groups, people with the most prevalent age-related conditions (e.g., controlled arterial hypertension, dyslipidemia, diabetes) were included. This ensures maximum representation from community-dwelling people,25 and is consistent with the World Health Organization's definition of ‘health’ as a state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity.26
Data collectionSociodemographic (age, sex), anthropometric (height and weight to compute body mass index – BMI), and general clinical (smoking habits, self-reported number of exacerbations and hospitalizations in the past year, self-reported medication and comorbidities, use of long-term oxygen therapy, and non-invasive ventilation) data were first collected. The severity of comorbid diseases was recorded and scored according to the Charlson Comorbidity Index.27 Physical activity level was assessed with the brief physical activity assessment tool.28 Lung function was assessed with a portable spirometer (MicroLab 3535, CareFusion, Kent, UK) according to standardized guidelines,29 and values from the forced expiratory volume in one second (FEV1) percentage predicted were used to classify people with COPD according to the GOLD grades (i.e., 1, 2, 3 or 4).2 GOLD groups (i.e., A, B, and E) were determined using the number of exacerbations and hospitalizations in the previous year and the modified Medical Research Council (mMRC) dyspnea questionnaire.2
Activity-related dyspnea was assessed with the mMRC.30 Impact of the disease was assessed with the COPD assessment test (CAT).31 Symptoms of anxiety and depression were measured with the hospital anxiety and depression scale.32 The Saint George's Respiratory questionnaire (SGRQ) and the World Health Organization quality of life questionnaire-bref were used to assess quality of life in people with COPD and healthy participants, respectively.33,34 Hand-grip and quadriceps muscle strength were measured on the dominant side during a maximum isometric voluntary contraction with a hand-grip (Jamar 12–0241 Lite, Fabrication Enterprises Inc., White Plains, NY, USA) and a hand-held dynamometer (microFET2, Hoggan Health, The best Salt Lake City, Utah, USA), respectively.35,36 The best of three measurements (less than 10% of variation) was used for analysis.
Functional capacity was assessed in all participants with the 5-STS and the 1-minSTS tests, in a standardized order. These tests are valid, reliable, and responsive measures in people with COPD.16,17 A straight-backed armless chair of 48 cm seat height, with a hard seat, stabilized against a wall was used to perform the tests.16,17 No encouragement was given during the performance of the tests. For the 5-STS, seated participants were asked to cross their arms at the chest and then to stand up all the way and sit down landing firmly, as fast as possible, for five times without using the arms.17 The best time of 3 trials was used for the analysis. For the 1-minSTS, participants were asked to sit with their hands stationary on the hips, without using the hands or arms to push against the chair, and were instructed to stand up all the way and sit down, touching the chair with their bottom when sitting, as many times as possible, in 1 min.16 Participants did not need to sit fully back on the chair.16 After 45 s, participants were told “you have 15 s left until the test is over”.16 Participants were allowed to use rest periods to complete the test. The number of completed repetitions was recorded. The best performance of 3 trials was used for the analysis. The percentage predicted values for the 5-STS and the 1-minSTS tests were calculated based on the reference equations.37
Matching processConsidering the known commonly reported characteristics of people with COPD (i.e., mostly male and aged 67 years on average),38,39 an effort was made to recruit a group of healthy controls composed mostly of men who were within the same age range. We explored if there were differences in age or sex between people with COPD and healthy controls. Sex was unbalanced between the two groups; hence, we explored the proportion of male/female in people with COPD and matched that proportion in the healthy controls. GraphPad Prism was used to randomly select the 75 healthy females (out of the 99 recruited) to be included in the analysis.
Data analysisStatistical analyses were performed using IBM SPSS Statistics version 28.0 (IBM Corporation, Armonk, NY, USA) and plots created using GraphPad Prism version 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). The level of significance was set at 0.05.
Descriptive statistics were used to describe the sample. Data are presented as mean (standard deviation [SD]), mean (95% confidence interval [CI]), median [interquartile range], or number (percentage). Normality of data distribution was explored with the Kolmogorov-Smirnov test. Independent t tests, Mann-Whitney U tests, and chi-squared tests were used to compare sociodemographic, anthropometric, and general clinical characteristics between people with COPD and healthy controls. The variables of interest (i.e., 5-STS and 1-minSTS) were further compared using analysis of covariance (ANCOVA) to control for age and sex as possible cofounding variables.
Comparisons between people with COPD, across the different GOLD grades (i.e., GOLD 1 & 2 vs 3 & 4), GOLD groups (i.e., GOLD A vs B vs E) and exacerbation history (i.e., GOLD A & B vs E), and healthy controls were performed with ANCOVA. When a significant difference was found, Bonferroni-adjusted pairwise comparisons were performed to determine which groups differed from the others.
The limit of normal (LN) for our population was established based on the performance of the healthy controls using the 90th percentile for the 5-STS (i.e., upper LN [ULN]) and the 10th percentile for the 1-minSTS (i.e., lower LN [LLN]).37 People with COPD were then classified according to their performance above/below the LN and comparisons between groups were explored with chi-squared tests, independent t tests, and Mann-Whitney U tests or mixed-model analysis variance (ANOVA) and Kruskal-Wallis tests followed by Bonferroni-adjusted pairwise comparisons, accordingly.
In people with COPD, correlations between the performance in the 5-STS and 1-minSTS tests and the other patient-reported and clinical measures were explored with Spearman's rank correlation coefficients (rs) and interpreted as: 0.00–0.19, very weak; 0.20–0.39, weak; 0.40–0.59, moderate; 0.60–0.79, strong; and 0.80–1.00, very strong.40
The ability of the different patient-reported and clinical measures to discriminate the performance of people with COPD in the 5-STS (i.e., above or below the ULN) and 1-minSTS tests (i.e., above or below the LLN) was explored with receiver operating characteristic analyses. The area under the curve was calculated and interpreted as: [0.5–0.6] poor discrimination, [0.6–0.7] acceptable discrimination, [0.7–0.8] excellent discrimination, and >0.9 outstanding discrimination.41
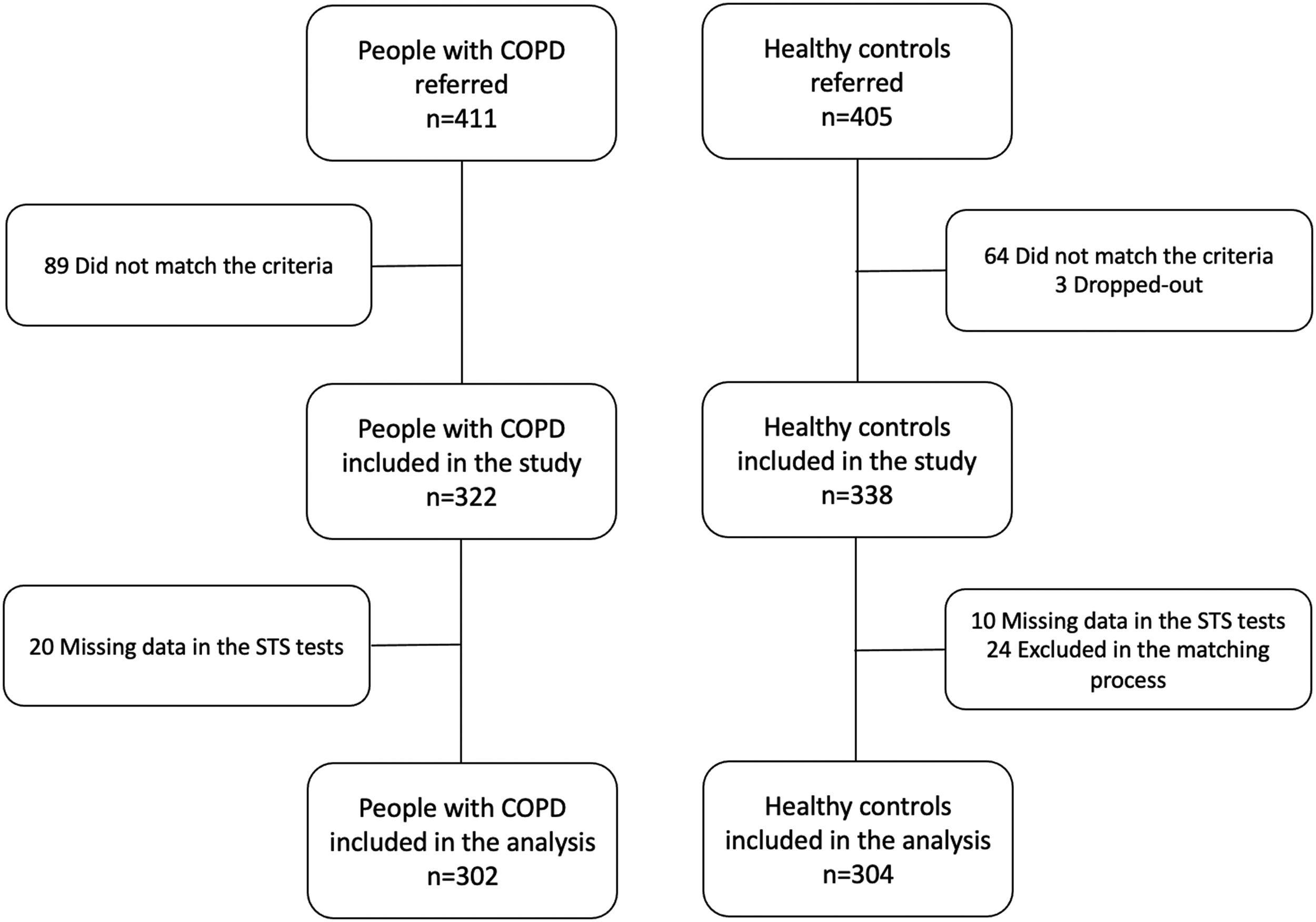
ResultsFour-hundred and eleven people with COPD and 405 healthy controls were referred to participate in the study. From these, 302 people with COPD [79.1% male; mean (SD) age: 67.5 (10.4) years] and 304 healthy controls [75.3% male; 66.2 (9.4) years] were included in the analysis (Fig. 1).
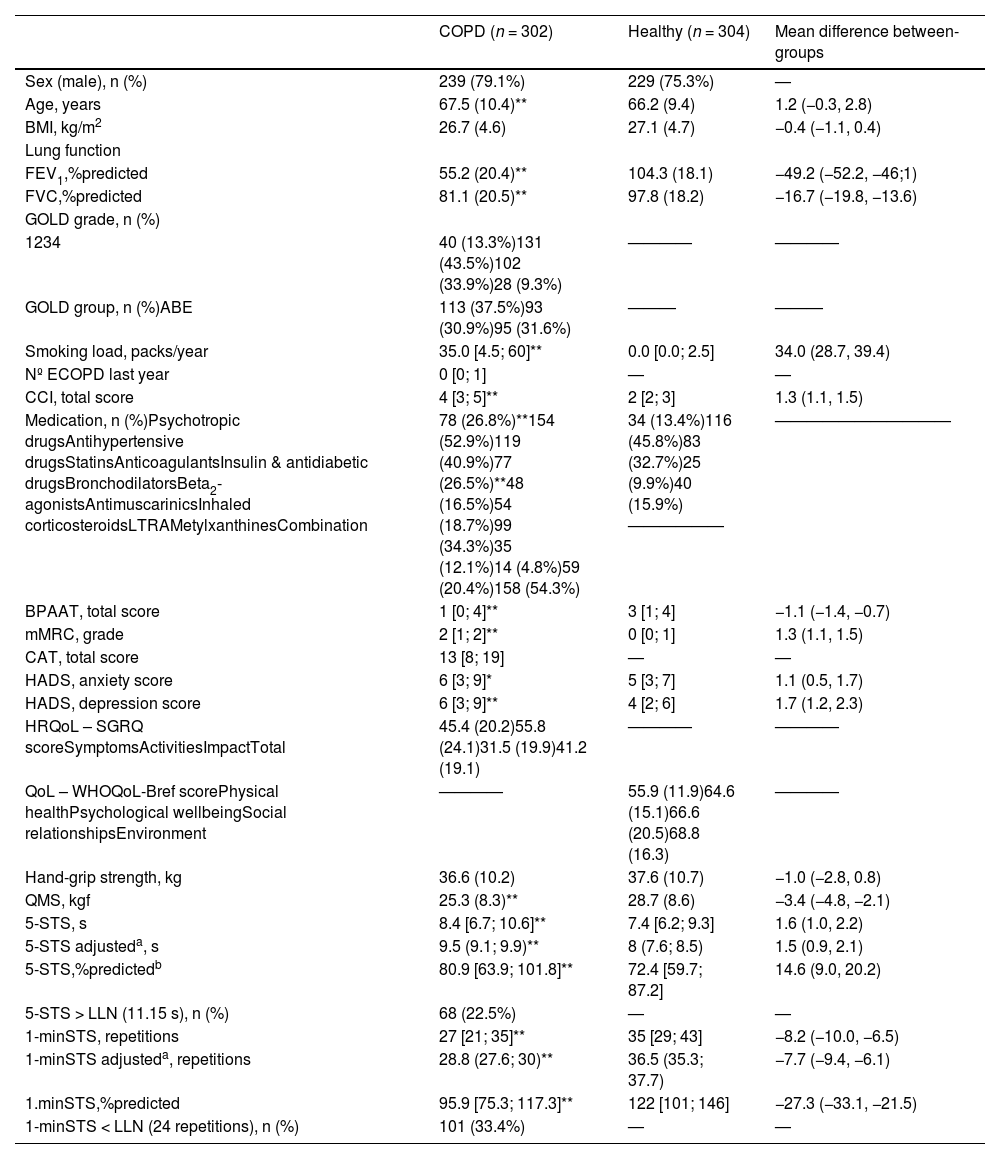
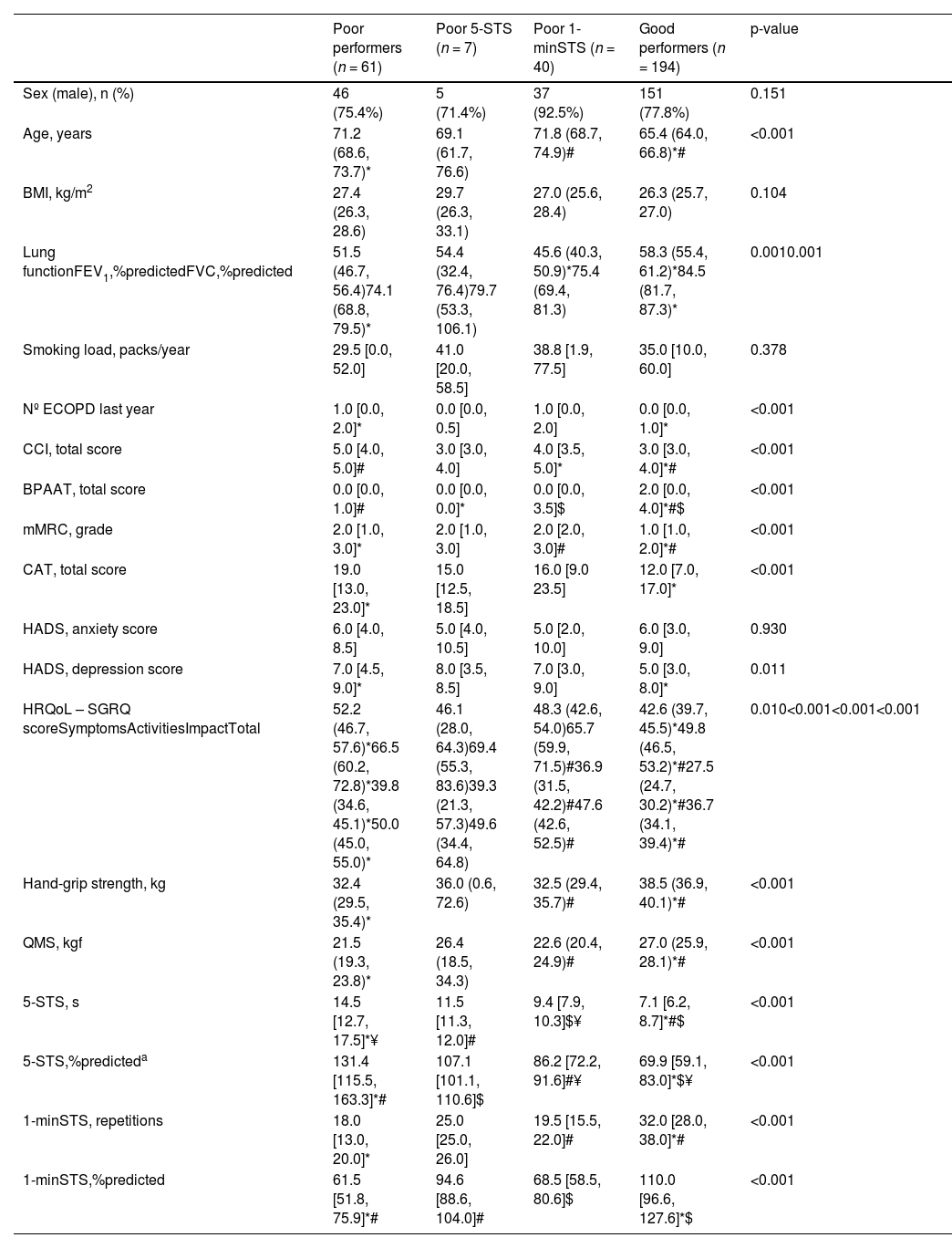
People with COPD and healthy controls were similar in terms of age and sex as they were matched for these variables, but also in BMI [mean difference (95% CI): −0.4 (−1.1, 0.4)]. People with COPD presented significantly higher comorbidity index, lower physical activity levels, higher symptoms of anxiety and depression, and worse quadriceps muscle strength than healthy people. FC impairment was present in 23% and 33% of people with COPD according to the 5-STS (i.e., 5-STS>ULN) and the 1-minSTS (i.e., 1-minSTS Table 1. Characteristics of people with COPD performing above/below the LN for each sit-to-stand test are presented in Supplementary online material (tables S1 and S2).
Characteristics of people with chronic obstructive pulmonary disease (COPD) (n = 302) and age-/sex-matched healthy controls (n = 304).
Data are presented as mean (standard deviation), mean (95 % confidence interval), median [1st quartile; 3rd quartile], or number (percentage), unless stated otherwise. aAnalysis corrected for age and sex. bIn this test, less time represents a better performance, hence going above 100 %predicted is not desired. *Denotes a statistically significant difference between groups (p < 0.05). **Denotes a statistically significant difference between groups (p < 0.001).
1-minSTS, 1-minute sit-to-stand test; 5-STS, 5-repetitions sit-to-stand test; BMI, body mass index; BPAAT, brief physical activity assessment tool; CAT, COPD assessment test; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; ECOPD, exacerbation of chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HADS, hospital anxiety and depression scale; HRQoL, health-related quality of life; LLN, lower limit of normality; LTRA, leukotriene receptor antagonists; mean diff, mean difference COPD – healthy; mMRC, modified Medical Research Council dyspnea questionnaire; QMS, quadriceps muscle strength; QoL, quality of life; SGRQ, Saint George's Respiratory questionnaire; WHOQoL-Bref, World Health Organization quality of life questionnaire-bref.
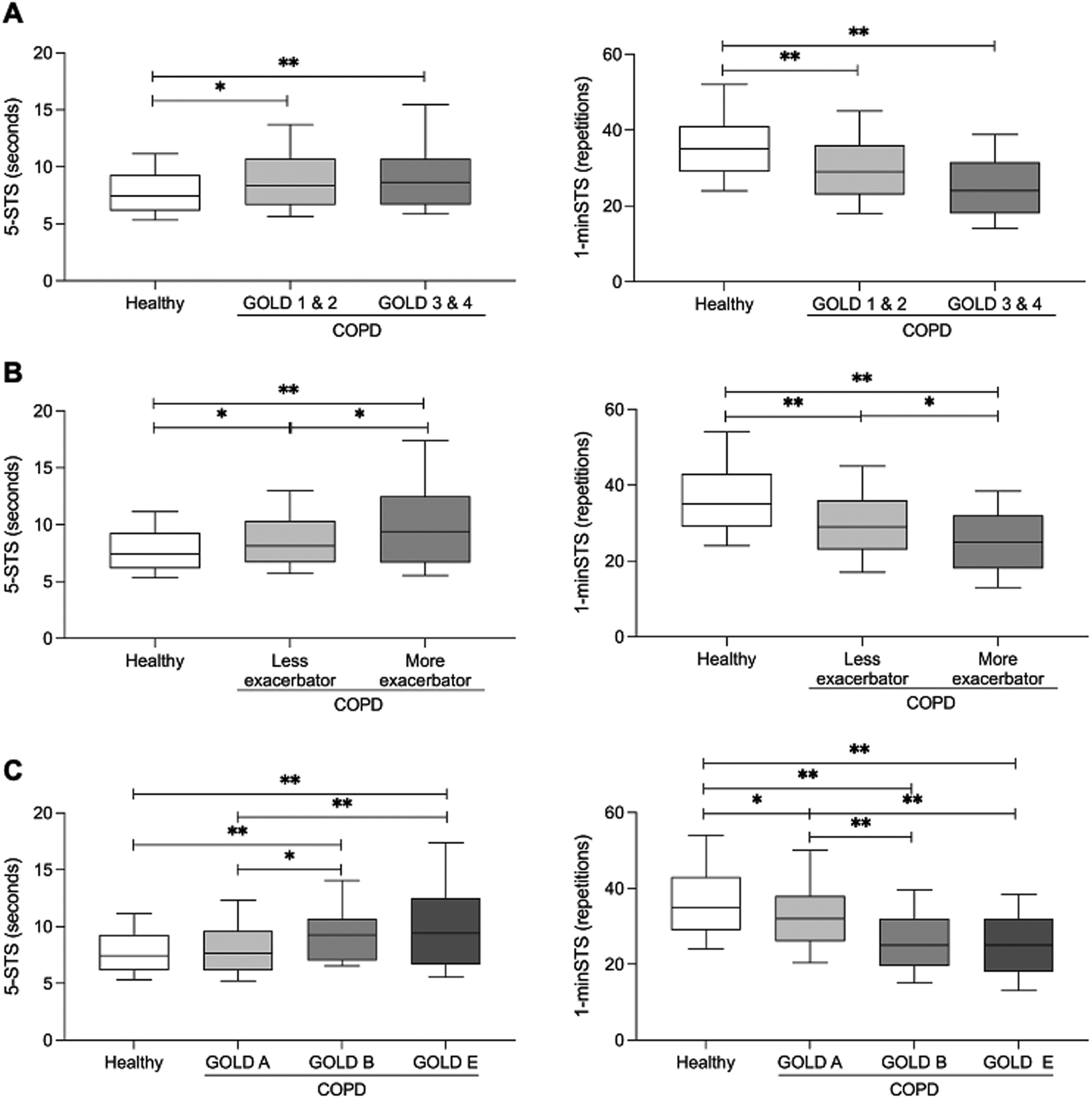
People with COPD performed worse than healthy people in the 5-STS and 1-minSTS, independently of their GOLD grade [GOLD 1 & 2 vs healthy: mean difference (95% CI) 5-STS 1.2 (0.4, 2.1), 1-minSTS −6.5 (−8.9, −4.1); GOLD 3 & 4 vs healthy: 5-STS 1.8 (0.9, 2.8), 1-minSTS −9.4 (−12, −6.8)] (Fig. 2A) or exacerbation history [less exacerbator vs healthy: mean difference (95% CI) 5-STS 1 (0.2, 1.8), 1-minSTS −6.5 (−8.7, −4.2); more exacerbator vs healthy: 5-STS 2.4 (1.4, 3.5); 1-minSTS −10.5 (−13.4, −7.5)] (Fig. 2B). A worse performance of more exacerbator people with COPD (i.e., GOLD E) in comparison to less exacerbator (i.e., GOLD A & B) was found in both tests [mean difference (95% CI) 5-STS: −1.4 (−2.5, −0.3); 1-minSTS (4.0 (0.9, 7.1)].
Boxplots of the median values with interquartile range in healthy controls vs people with chronic obstructive pulmonary disease (COPD) on the 5-repetitions sit-to-stand test (5-STS, left side) and 1-minute sit-to-stand test (1-minSTS, right side). A) Comparisons between healthy controls and people with COPD across Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric grades 1 & 2 and 3 & 4. B) Comparisons between healthy controls and people with COPD according to exacerbation history. Less exacerbator – GOLD groups A & B, More exacerbator – GOLD group E. C) Comparisons between healthy controls and people with COPD across GOLD groups (i.e., A, B, E). GOLD group A – Less symptomatic, Less exacerbator; GOLD group B – More symptomatic, Less exacerbator; GOLD group E – More exacerbator, independent of symptomatic level. The line in the middle of the box represents the median, the box extends from the 25th percentile to the 75th percentile, and the whiskers are drawn down to the 10th percentile and up to the 90th percentile. *p < 0.05; **p < 0.001.
Regarding GOLD groups, only more symptomatic people with COPD (i.e., GOLD B) and exacerbators (i.e., GOLD E) presented a lower performance in the 5-STS [GOLD B vs. healthy: mean difference (95% CI) 1.8 (0.7, 3.0); GOLD E vs healthy: 2.4 (1.3, 3.6)] (Fig. 2C). People with COPD performed worse than healthy people in the 1-minSTS, independently of their GOLD group [GOLD A vs healthy: mean difference (95% CI) −3.4 (−6.4, −0.4); GOLD B vs healthy: −10.3 (−13.5, −7.1); GOLD E vs healthy: −10.5 (−13.7, −7.3)]. Additionally, more symptomatic people with COPD (i.e., GOLD B) and exacerbators (i.e., GOLD E) performed worse than less symptomatic and exacerbator people (i.e., GOLD A) in the 1-minSTS [GOLD A vs GOLD B: mean difference (95% CI) 6.9 (3.1, 10.7); GOLD A vs GOLD E: 7.1 (3.3, 10.9)] (Fig. 2C).
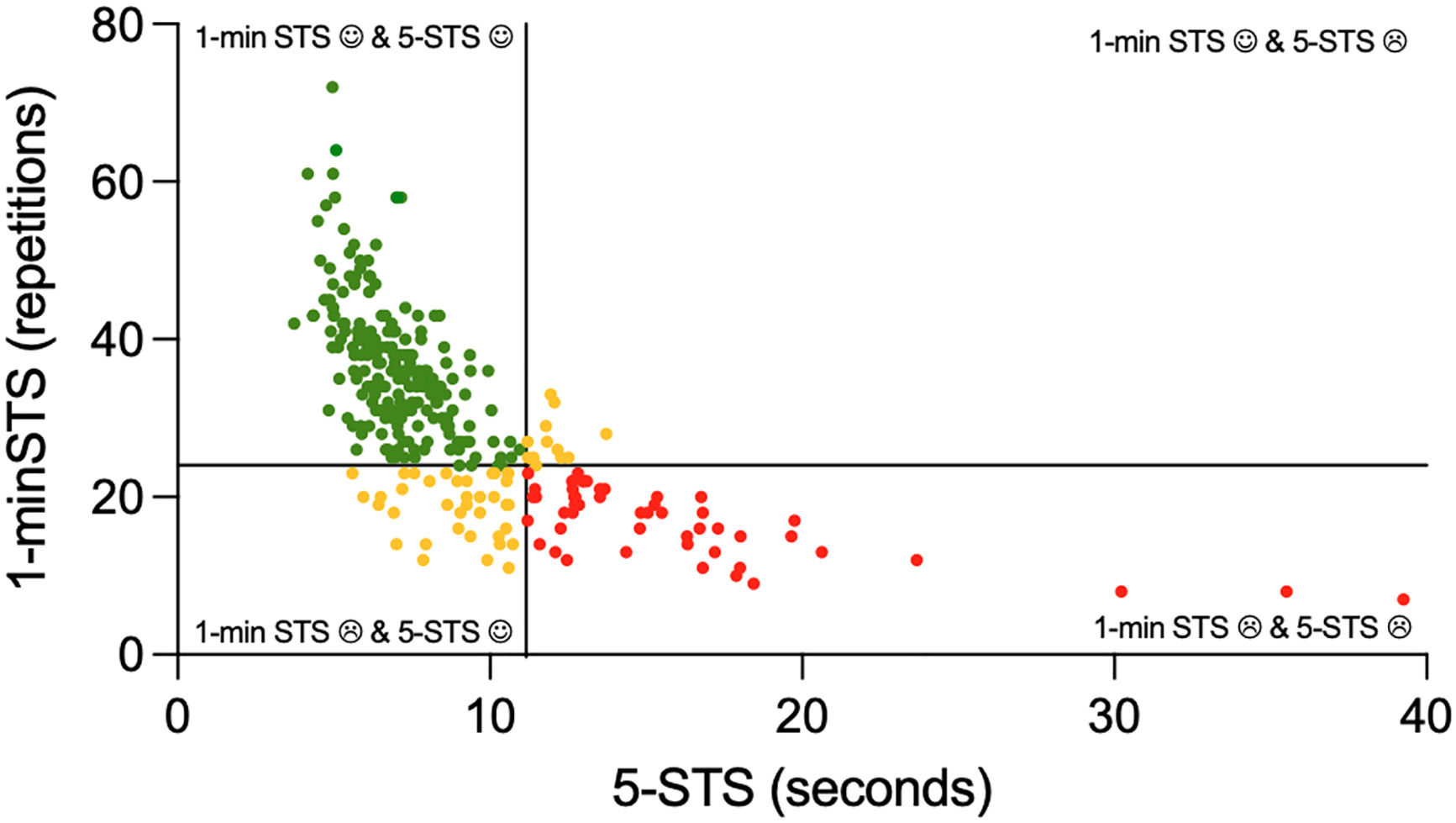
When combining the performance in the 5-STS with the performance in the 1-minSTS, most people with COPD (64%) were good performers in both tests, 20% were poor performers in both tests, 13% had a low performance only in the 1-minSTS, and 2% had a low performance only in the 5-STS (Fig. 3).
Distribution of people with chronic obstructive pulmonary disease according to their performance in the 5-repetitions sit-to-stand (5-STS) and 1-minute sit-to-stand (1-minSTS) tests. The vertical line represents the upper limit of normal of 11.15 s for the 5-STS. The horizontal line represents the lower limit of normal of 24 repetitions in the 1-min STS. Green points represent the good performers in both tests, red points represent the poor performers in both tests and yellow points represent the poor performers in only one of the tests.
Poor performers on both tests were older, had higher exacerbation history, comorbidity index, activity-related dyspnea, and impact of the disease, and decreased physical activity levels, quality of life, and muscle strength compared to good performers in both tests (Table 2).
Characteristics of people with chronic obstructive pulmonary disease (COPD) according to their combined performance in the 5-repetitions sit-to-stand (5-STS) and 1-minute sit-to-stand (1-minSTS) tests.
Data are presented as mean (95 % confidence interval), median [1st quartile; 3rd quartile], or number (percentage), unless otherwise stated. The symbols (*, #, $, ¥) identify the comparisons that are significantly different. Poor performers, people with chronic obstructive pulmonary disease (COPD) performing above the upper limit of normal (ULN, 11.15 s) in the 5-repetitions sit-to-stand test (5-STS) and below the lower limit of normal (LLN, 24 repetitions) in the 1-minute sit-to-stand test (1-minSTS); Poor 5-STS, people with COPD performing above 11.15 s in the 5-STS and equal or above 24 repetitions in the 1-minSTS; Poor 1-minSTS, people with COPD performing equal or below 11.15 s in the 5-STS and below 24 repetitions in the 1-minSTS; Good performers, people with COPD performing equal or below 11.15 s in the 5-STS and equal or above 24 repetitions in the 1-minSTS. aIn this test, less time represents a better performance, hence going above 100 %predicted is not desired.
1-minSTS, 1-minute sit-to-stand test; 5-STS, 5-repetitions sit-to-stand test; BMI, body mass index; BPAAT, brief physical activity assessment tool; CAT, COPD assessment test; CCI, Charlson comorbidity index; COPD, chronic obstructive pulmonary disease; ECOPD, exacerbation of chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HADS, hospital anxiety and depression scale; mMRC, modified Medical Research Council dyspnea questionnaire; QMS, quadriceps muscle strength; SGRQ, Saint George's Respiratory questionnaire.
Very weak to weak correlations were found between the results in the 5-STS or 1-minSTS and the different patient-reported and clinical measures in people with COPD (Supplementary online material, Table S3). Only the mMRC (rs=–0.42), SGRQ activities score (rs=–0.45), and SGRQ total score (rs=–0.42) presented significant negative moderate correlations with the 1-minSTS.
In people with COPD, only poor to acceptable discrimination was found for the different patient-reported and clinical outcomes in predicting performance (i.e., ≤ or >11.15 s) in the 5-STS (Supplementary online material, Table S4). For the 1-minSTS, poor to excellent discrimination was found among the different patient-reported and clinical outcomes, the SGRQ activities score was the best discriminator of performance (i.e., < or ≥24 repetitions) in the 1-minSTS (Supplementary online material, Table S4).
DiscussionThis study found that: i) 23–33% of people with COPD exhibit impaired FC; ii) people with COPD have decreased FC in comparison to healthy controls, independently of their severity of lung function obstruction, exacerbation history, or symptomatic level; iii) in the 1-minSTS, a higher exacerbation history or symptomatic level results in further FC decline; iv) people with FC impairment are older, have a higher exacerbation history, comorbidities, activity-related dyspnea and impact of the disease, and lower physical activity levels, muscle strength, and quality of life; and v) other patient-reported and clinical outcomes are mostly poorly correlated to measures of FC and show limited discriminative ability for these measures.
Similarly to previous findings on physical activity, quadriceps muscle strength, and limb muscle function,42-45 a decline in FC is present since the early stages of COPD. This decline is independent of the level of lung function obstruction, reinforcing that lung function parameters cannot be used to predict FC.46 It also suggests that functional impairment is an early extra-pulmonary feature of COPD and, therefore, a comprehensive assessment and management of this treatable trait (i.e., functional impairment) is needed.
Prevalences of functional impairment ranging from 22.3 to 64.4%,42,43,47-49 depending on the outcome measure, have been previously reported in people with COPD. In this study, a prevalence of FC impairment of 23–33% was found, which falls within that range. Several factors might contribute to this wide range in prevalences. First, studies include different populations of people with COPD, with different symptomatic levels and exacerbation history, parameters known to impact FC.50-52 Moreover, the use of different reference values and/or equations and different methodologies to establish the limit of normality influences the determination of the presence/absence of impairment.53-56 Lastly, the use of different outcome measures to assess FC also results in different classifications regarding the presence/absence of impairment.47 This further highlights the need for an accurate identification of the presence/absence of this trait to guide treatment strategies.
Interestingly, a higher prevalence of functional impairment was found using the 1-minSTS in comparison to the 5-STS, and the 1-minSTS was the only test able to discriminate between people with COPD with different exacerbation history and symptomatic levels. Moreover, only 2% of people with COPD presented impairment in the 5-STS without also showing impairment in the 1-minSTS. Thus, it seems that the 1-minSTS is a more suitable test to accurately discriminate amongst people with stable COPD. In fact, the literature has shown that the 1-minSTS is a more stressful test to the cardiorespiratory system (inducing a similar stress to the cardiopulmonary exercise test) and demands a higher physical effort than the 5-STS.1,57 The 5-STS is part of the screening measures to identify sarcopenia, a good measure to identify frailty, and a predictive factor for hospitalization due to exacerbation.19,58-62 It is, therefore, likely that the 5-STS is more suitable for more frail contexts, such as during exacerbations and hospitalizations, where a higher symptomatic burden and impact of the disease is experienced.52 Furthermore, although both tests are measures of FC, the 5-STS has been more associated with muscle strength and power, coordination, and posture control; while the longer duration of the 1-minSTS has been more related with muscle endurance and higher hemodynamic and symptomatic demands, contributing to the use of anaerobic processes and muscle fatigability.1,15,63,64 Considering the specificities of training, it is therefore likely that individuals with impairments in the 5-STS may benefit from resistance training, individuals with an impaired 1-minSTS may benefit from endurance training, and individuals with impairments in both tests will need a program combining resistance and endurance training.65,66
People with functional impairment were found to be older, have a higher exacerbation history, comorbidities, symptoms, and impact of the disease, and lower physical activity levels, muscle strength, and quality of life. Similar characteristics in people with functional impairment have been previously reported.7,67,68 Because most of these characteristics are preventable and modifiable, attention to interventions in this population should be given. In fact, different stakeholders have stated that people with functional impairment should be prioritized to pulmonary rehabilitation,69 and these programs have shown to be effective in improving the FC of people with COPD.47,70,71 Nevertheless, there is a large number of non-responders on FC and the effects of pulmonary rehabilitation are not sustained after the intervention.47,70 Thus, future studies should investigate how to personalize pulmonary rehabilitation to maximize and sustain its effects on FC.
Limited discriminative ability and overall weak correlations were found between the different patient-reported and clinical outcomes and the sit-to-stand tests, which is consistent with previous findings.37,72,73 This emphasizes the complexity of COPD and how different measures reflect different constructs, reinforcing the need to perform comprehensive functional assessments.
This study has some strengths and limitations that need to be acknowledged. We included a relatively large sample, which is representative of our population considering a 95% confidence interval and a 5% margin of error. Moreover, we collected data across different settings and geographic regions, reinforcing the representativeness of real-world data. Functional impairment in people with COPD was defined based on the results of a matched healthy Portuguese population instead of other population-based reference values or predictive equations, which also strengthens our results. Nevertheless, our sample has an unbalanced proportion of male/female and is mostly composed of people with moderate to severe COPD. Although these characteristics are commonly reported in this population,38,39 we acknowledge that it might limit the generalization of our findings to all people with COPD. Additionally, being healthy was self-reported by participants according to how they felt. Although there was no formal medical appointment, this is consistent with the World Health Organization's definition of health,26 and the satisfactory results (within normality) obtained by the healthy controls attest their health status. Lastly, functional status is a comprehensive outcome that can be assessed with several measures,3,6 thus the use of only sit-to-stand tests, which focus on the assessment of FC, might have limited the identification of impairment and characterization of the population. Future studies should try to integrate a combination of measures of FC (e.g., 6MWT, sit-to-stand, stair climbing) with measures of functional performance (e.g., accelerometer, Clinical COPD questionnaire functional status domain, Canadian occupational performance measure), or use combined measures of capacity/performance (e.g., Glittre activities of daily living, Londrina activities of daily living) if time and space constraints allow it.3
ConclusionPeople with COPD exhibit decreased FC, independently of the severity of lung function obstruction, exacerbation history, or symptomatic level. This decline in FC might be better reflected by the 1-minSTS than the 5-STS (as a slightly larger proportion of individuals presented impairment in the 1-minSTS than in the 5-STS) and affects 23–33% of people with stable COPD. Because impaired FC is a treatable trait not accurately reflected by other patient-reported and clinical outcomes, a comprehensive assessment and management of this early extra-pulmonary feature of COPD is needed.
The authors would like to acknowledge the support of all the individuals who agreed to participate in this study. We also want to thank all the institutions, clinicians, and staff involved in the recruitment process. This work was funded by Fundação para a Ciência e a Tecnologia (FCT) under the PhD grant SFRH/BD/147200/2019 & COVID/BD/153335/2023. This work was also supported by Programa Operacional de Competitividade e Internacionalização – POCI, through Fundo Europeu de Desenvolvimento Regional - FEDER (POCI-01-0145-FEDER-028806), FCT (PTDC/SAU-SER/28806/2017) and under the project UIDB/04501/2020. Patrícia Rebelo (PhD grant SFRH/BD/148738/2019) and Sara Souto-Miranda (PhD grant SFRH/BD/146134/2019) are funded by FCT.