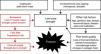
Osteoporosis and related fragility fractures are a global public health problem in which pharmaceutical agents targeting bone mineral density (BMD) are the first line of treatment. However, pharmaceuticals have no effect on improving other key fracture risk factors, including low muscle strength, power and functional capacity, all of which are associated with an increased risk for falls and fracture, independent of BMD. Targeted exercise training is the only strategy that can simultaneously improve multiple skeletal and fall-related risk factors, but it must be appropriately prescribed and tailored to the desired outcome(s) and the specified target group.
ObjectivesIn this review, we provide an overview of the general principles of training and specific loading characteristics underlying current exercise guidelines for the prevention of osteoporosis, and an update on the latest scientific evidence with regard to the type and dose of exercise shown to positively influence bone mass, structure and strength and reduce fracture risk in postmenopausal women.
Osteoporosis is a global clinical and public health problem because it is associated with an increased risk for fragility fractures which can lead to pain, disability, loss of functional independence and increased morbidity and mortality. It is more common in women than men, with the prevalence increasing markedly after the menopause. Approximately 30% of all postmenopausal women in Europe and the United States are reported to have osteoporosis, and at least 40% of these women will sustain one or more osteoporotic fractures in their remaining lifetime.1,2 After an initial fracture the risk for subsequent fracture more than doubles in the next 6 to12 months, and persists for up to 10 years.3,4 Furthermore, around one in three people will die within 12 months of a hip fracture, 40% will be institutionalized or unable to walk independently, and 60% will still require assistance a year later.5,6 Pharmaceutical agents targeting bone mineral density (BMD) are the first line of treatment for osteoporosis because they reduce the risk of fractures by approximately 20–60% depending on the agent used, patient population and adherence to the medication.7 However, it is estimated that 80% of postmenopausal women that sustain a fragility fracture fail to receive appropriate follow-up treatment,8 and for those on medical treatment adherence is often poor which may be related to safety concerns (e.g. osteonecrosis of the jaw).9 Pharmaceuticals also have no effect on other key fracture risk factors, such as muscle strength, muscle power, dynamic balance, coordination and overall functional performance, all of which have been associated with an increased risk for falls and fracture (Fig. 1).10 Exercise training is the only strategy that can improve all modifiable fracture risk factors (bone strength, fall risk, fall impact), but it must be appropriately prescribed and adherence needs to be maintained. This review provide an overview of: (1) the key principles of training and characteristics of loading underlying current exercise guidelines for the prevention of osteoporosis and fragility fractures, and (2) an update on the latest evidence with regard to the type and dose of exercise training shown to influence bone mass, structure and strength and reduce fracture risk in postmenopausal and older women. The focus is on the prevention, rather than management, of osteoporosis and fractures.
Key loading characteristics and training principles to optimize bone healthBone is a dynamic tissue that responds to changes in mechanical loads by altering its mass, structure and/or strength, controlled via a negative feedback system, in order to withstand future loads to prevent fracture. Our understanding of the key loading characteristics necessary to stimulate an adaptive skeletal response has been informed by the findings from many animal studies which have shown that bone responds to: (1) dynamic intermittent rather than static loads11; (2) loads that are high in magnitude and applied rapidly12,13; (3) loads that are applied in unusual or diverse loading directions or patterns14,15; and (4) relatively few loading cycles (repetitions), if an adequate load intensity is achieved.15,16 This is because bone cells desensitize to repetitive loading, and thus the capacity of bone to respond to continual loading diminishes over time or with increasing repetitions. For instance, there is evidence that short bouts of loading interspersed with periods of rest are more osteogenic than the same number of loads performed continuously.17 Collectively, these findings are important as they have guided the development of clinical exercise prescription guidelines for the prevention and management of osteoporosis.18 However, the American College of Sports Medicine (ACSM) has also recommended that the general training principles aimed at improving training adaptations should also be considered when designing any exercise program to optimize bone health.19
1. Principle of Specificity: skeletal adaptations to loading are site-specific and not systemic in nature. Thus, the prescription of exercise must include targeted activities that are known to directly (via gravitational loading) or indirectly (via the action of muscle pulling on bone) load the skeletal site(s) of interest, particularly the hip, spine and wrist, which are the most common fracture sites. The importance of this principle is demonstrated by the findings from a simple 2-year back extension strengthening exercise program in postmenopausal women using a weighted backpack (10 repetitions, 5 days per week) which improved spinal extensor muscle strength and was associated with greater spinal bone density and fewer vertebral fractures 8 years later compared to controls.20 Similarly, a high impact jumping exercise intervention performed 2–3 times per week in postmenopausal women was found to improve proximal femur, but not lumbar spine, BMD after 12 months.21
2. Principle of Progressive Overload: the loads or strain imparted to bone via gravitational or muscle forces must exceed the typical loading patterns encountered during everyday activities, and as bone adapts the loading stimulus must be increased progressively. This principle is supported by Frost's ‘mechanostat’ theory, which proposes that bones have a set-point or threshold level of adaptation called the minimum effective strain (MES), such that loads (strains) above (or below) this ‘set-point’ will stimulate bone formation (or resorption) leading to an increase (or decrease) in bone strength.22 Although the magnitude of loads imparted to bone is central to this theory, the pattern (distribution), rate, number and frequency of loading are also key overload training characteristics to consider when designing an exercise program to improve bone health.
3. Principle of Reversibility: any positive skeletal adaptations resulting from exercise training will be progressively lost once the program or stimulus is discontinued.23–25 However, an important question that requires further study is whether there is a minimal dose of exercise needed to retain any initial exercise-induced skeletal gains. The findings from a 16-year non-randomized study involving a multi-modal exercise program in early postmenopausal women with osteopenia found that at least two sessions per week was the minimum effective dose to positively influence bone over the long-term.26 However, these results may not be generalizable to other populations, exercise modalities or protocols, and thus further studies are needed to evaluate whether there is a minimum dose of exercise to maintain any initial exercise-induced skeletal adaptations in older adults.
4. Principle of Initial Values: the greatest changes in bone in response to loading will typically occur in those with the lowest initial bone mineral density.27 However, the initial values effect may also closely reflect the principle of progressive overload, such that smaller or weaker bones will experience greater strain than larger or stronger bones exposed to the same absolute load. Therefore, if the relative intensity or pattern of loading is of a sufficient magnitude and rate or differs from everyday movement patterns, then bones should adapt accordingly, regardless of the initial values.
5. Principle of Diminished Returns: following any initial exercise-induced skeletal adaptation subsequent gains are likely to be slow and modest with a similar loading regimen. This is consistent with the “Principle of Cellular Accommodation” which proposes that bone cells initially respond strongly to a given load of sufficient magnitude, rate or frequency, but this response will eventually phase out as the cells learn or accommodate to the new loads.28 This is highlighted by the findings from several exercise interventions over 12–18 months which reported that the greatest changes in BMD occurred during the initial 5–6 months.29,30 However, others have reported a linear increase in BMD with continued exercise training,31,32 which may relate to the fact that a progressive exercise program was implemented that resulted in sustained overload and thus ongoing skeletal adaptations. This implies that the principle of diminished returns is influenced by the principles of initial values and progressive overload, that is, following any initial skeletal adaptations bone may experience less strain if the loads remain unchanged.
It is on the basis of these key loading characteristics and training principles that many human intervention trials have been conducted to evaluate the effects of various exercise modalities and training doses on bone in postmenopausal of various ages. However, other important factors to consider when prescribing exercise for bone health is that the response time of bone to loading is slow because the typical bone remodelling cycle lasts 3 to 8 months. Thus interventions must last a minimum of 6 to 9 months (preferably 12 to 24 months) to detect any measurable or ‘true’ physiological skeletal changes beyond the normal bone remodelling transient. Longer follow up periods (≥24 months) may be required to detect changes in (p)QCT bone structural properties in postmenopausal women.33 It is also important to note that the mechanosensitivity of bone diminishes with age and any exercise-induced changes in bone density after menopause are typically modest (1–3%). However, even a maintenance in BMD may be clinically relevant given that the average rate of bone loss is around 2–4% per year in the first 5 to 10 years after menopause and 1–2% per year thereafter.34 The findings from pharmaceutical trials also indicate that an increase (or difference over placebo) of ∼2–4% in DXA BMD alone over 1-year is associated with a 42–59% fracture risk reduction.35 Thus, the seemingly modest gains in BMD observed following exercise intervention trials in older adults are likely to be clinically relevant.
Exercise for the prevention of fragility fracturesFrom a clinical perspective, an important question that remains uncertain is whether exercise training can prevent fragility fractures. To date, there have been no long-term and adequately powered randomized controlled trials (RCT) to address this question as it would require a sample size of approximately 7000 high risk persons to be followed for at least 5 years.36 At present, the highest level of evidence is from a systematic review and meta-analysis of 10 exercise controlled trials (with and without randomization) in adults aged 45 years and older which found that exercise training reduced overall fracture number (10 trials) by 51% [relative risk (RR), 0.49 (95% confidence interval (CI): 0.31–0.76)] and vertebral fracture number (three trials) by 44% [RR, 0.56 (95% CI: 0.30–1.04)].37 However, these findings must be interpreted with caution due to the small number of studies and evidence of publication bias. A subsequent meta-analysis of 15 RCTs found that exercise training reduced the risk of fall-related fractures by 40% in adults aged 50 years and over [RR 0.60 (95% CI: 0.45–0.84)].38 This is important because around 90% of all hip fractures result from a fall.
It is beyond the scope of this paper to review the evidence related to the role of exercise for falls prevention, but meta-analyses of exercise RCTs with falls as the outcome have found that programs including challenging balance training for at least 3h per week or reactive and volitional stepping training can reduce the risk of falls by approximately 39% and 50%, respectively, in older people.39,40 However, falls prevention programs have been shown to have little or no effect on BMD.41 Therefore, the design of any exercise program to prevent fragility fractures must include activities that will specifically target bone as well as fall-related risk factors, such as muscle weakness, reduced muscle power, poor balance and slow walking speed.
Exercise for the prevention of osteoporosis in postmenopausal womenWhile current clinical practice guidelines for the prevention and management of osteoporosis recommend exercise training as an effective approach to maintain bone mass or slow bone loss throughout the postmenopausal years and into old age,18 not all forms or doses of exercise training are equally effective for eliciting a positive skeletal response. The current evidence to support exercise prescription guidelines in terms of the optimal type and dose [magnitude, rate, number of repetitions, frequency (sessions or days per week)] for bone health is summarized below.
Walking and others forms of aerobic exerciseRegular walking for leisure in isolation and other forms of low or non-impact aerobic activities such as cycling and swimming have been shown to have little or no effect on preventing age-related bone loss in postmenopausal women.42,43 This can be explained by the fact that these activities typically impart low level (or customary) loads (strain) on bones that are not sufficient to exceed the required threshold for skeletal adaptation. However, a meta-analyses of 11 randomized, non-randomized and prospective observational studies in men and women aged 45 years or older reported that water-based exercise training reduced age-related bone loss at the hip and lumbar spine, but land-based exercises were more effective for enhancing bone health.44 However, these findings must be interpreted with caution due to the low quality of available studies and the inclusion of non-randomized and observational studies.
Others have reported that brisk walking at intensities around 75% or greater of maximum oxygen uptake,45 walking with a weighted vest46 or walking in combination with others forms of exercise (jogging, stair-climbing, stepping)47,48 can provide some protection against bone loss. However, frequent walking or the inclusion of walking in an exercise program for sedentary or frail elderly has been associated with an increased risk of falls and fracture in some studies.49,50 Therefore, despite the benefits of walking on aerobic fitness, body composition and cardiometabolic health, the current evidence does not support walking as a single intervention for the prevention of osteoporosis, falls or fractures.
Progressive resistance trainingProgressive resistance training (PRT) is recommended as an effective strategy to increase or maintain BMD in postmenopausal women because it can place a diverse range of loads (strain) on bone via the direct pulling action of muscles (joint reaction forces) and/or by the increased effect of gravity acting on bone when the skeleton supports heavier weights (ground reaction forces).51 However, there are mixed findings with regard to the effects of PRT on hip and spine BMD in postmenopausal women,52,53 despite marked improvements in muscle mass and strength. This is likely to be attributed to a number of factors related to the five general training principles, including the prescription of a low or inadequate training dose or intensity, lack of exercise specificity and lack of training progression, in addition to the inclusion of healthy women with normal BMD and/or inadequate sample sizes.
Resistance training programs which have been shown to maintain or improve BMD in older women have typically incorporated moderate to high intensity loads (2–3 sets of 8–12 repetitions at 70–85% of maximal muscle strength) that increased progressively over time and targeted large muscles crossing the hip or spine and which were prescribed at least 2–3 times per week.18,51 Greater skeletal benefits in response to PRT have been observed at the lumbar spine than at the hip, which could be attributed to the fact that resistance exercises may not impart sufficient loads (strain) across the proximal femur to elicit a positive skeletal response.54 Despite these mixed results, PRT is the most effective strategy to improve various non-skeletal risk factors for fracture, particularly skeletal muscle mass, size and strength, and thus should form the basis of any exercise programs designed to reduce fracture risk.
High-velocity power trainingSkeletal muscle power, or the ability to produce force quickly, decreases earlier and more rapidly with advancing age than muscle mass and strength, which has been largely attributed to the age-related loss in type II fast twitch muscle fibres. In addition, people with osteoporosis have been shown to have preferential and diffuse type II muscle fibre atrophy, and this has been related to the degree of bone loss in older women.55 As a result, there has been interest in the role of high-velocity (power) resistance training, which involves rapid concentric muscle contractions that may induce high strain rates on bone, as an approach to optimize bone health. One 2-year study in 53 postmenopausal osteopenic women found that twice weekly power training maintained hip and lumbar spine BMD compared to traditional PRT after 12 months, and these benefits persisted at the spine after 2 years.56,57 While further studies are needed to confirm these findings, this type of training has also been shown to be more effective than traditional PRT for improving functional performance (chair rising time and stair climbing ability) in older adults,58 which is important for falls and subsequent fracture prevention.
Weight-bearing impact exerciseShort bouts of weight-bearing impact exercise (3–5 sets of 10–20 jumps, 4–7 days per week) that include moderate to high magnitude loads (>2–3 times body weight) and multidirectional movement patterns are promoted to maintain or prevent bone loss in older adults.18 However, the strength of the evidence from RCTs to support the efficacy of this mode of training on bone health in postmenopausal women is mixed.59,60 Several exercise trials incorporating 2–3 sessions per week of progressive stepping and jumping training21 or weighted vest jumping (average 52 jumps per session)61 reported improvements or a maintenance in proximal femur BMD in postmenopausal women compared to controls. In contrast, a 12-month trial in which postmenopausal women performed 50 vertical jumps (4 times body weight) 6 days per week observed no significant effect on proximal femur or lumbar spine BMD, despite benefits in premenopausal women.62 It has been proposed that the blunted osteogenic response in postmenopausal women may be due to depleted oestrogen levels. In part support of this notion, a meta-analysis of six intervention trials in postmenopausal women aged 52 to 68 years found that combined hormone replacement therapy (HRT) and exercise was associated with greater improvements in femoral neck and lumbar spine BMD than exercise alone.63
The mixed findings with regard to the effects of weight-bearing exercise on bone may be related to differences in the exercises prescribed and/or technique used, the non-progressive nature of some programs, a failure to incorporate multi-directional or novel loading activities and/or compliance issues associated with other comorbidities (e.g. pain from osteoarthritis). Indeed, the findings from a meta-analysis of exercise interventions with different impact loading characteristics reported that RCTs involving odd-impact (exercises performed in different directions) protocols (as well as combined impact and resistance training programs) were effective at improving lumbar spine and femoral neck BMD, but there was significant heterogeneity.59
To better inform the prescription of exercise for bone health in humans a number of recent studies have used computational modelling techniques to evaluate the in vivo bone tissue strains within the proximal femur under various loading conditions, and to determine which muscles are more important for loading specific skeletal areas that are prone to fracture.54,64,65 For instance, a study in 14 postmenopausal women (mean age 64 years) which evaluated the osteogenic potential of different exercises at various intensities to load the femoral neck found that hopping, running (5–9km/h) and fast walking (5–6km/h) resulted in higher compressive and tensile strains compared to walking at 4km/h, which was considered the minimal level for bone preservation.54 In addition, at the superior region of the femoral neck, which is an area of focal weakness prone to fracture, fast walking, running and hopping were all found to impart compressive and tensile strains of a sufficient magnitude likely to induce an osteogenic response. In contrast, all resistance training exercises (hip extension and flexion, hip abduction and adduction) at 40–80% of maximum muscle strength only induced strains that were equivalent to or lower than that reported for walking at 4km/h, which may explain the mixed findings in terms of the effects of resistance training on hip BMD in older women.
Another study in 20 postmenopausal women also used computational modelling to estimate the strain distribution patterns across the proximal femur for walking (normal and fast pace), stair ascent and descent, and a vertical jump, and the specific muscles loading the femoral neck during each activity.64 There were a number of key findings from this study which can be used to guide the design of future exercise programs for older adults: (1) the trochanteric region experienced the highest strains for all activities, which is likely due to the muscle attachments at this site; (2) the distribution of strain varied across the proximal femur for the different exercises, with stair ambulation and the vertical jump producing higher strains in the anterior and superior aspects of the femoral neck (the key areas prone to weakness and fracture) relative to walking; (3) the gluteal muscles (hip extensors) were responsible for inducing strains in the femoral neck during stair ambulation and jumping, in contrast to walking in which the iliopsoas muscle (hip flexor) induced strains, and (4) the ground reaction forces associated with each exercise were closely associated with the level of strain during each task, which suggests that they can provide a surrogate indicator of the potential for a given exercise to load the femoral neck. A summary of peak vertical ground reaction forces of common weight-bearing activities that could be incorporated into an exercise program for postmenopausal and older women is provided in Table 1.
Peak vertical ground reaction forces (normalized to body weight) for a range of weight-bearing impact activities.a
| Activity | Peak vertical ground reaction force (relative to body weight) |
|---|---|
| Lunge | 1.1 |
| Walking | 1.2 |
| Side lunge | 1.2 |
| Marching on the spot | 1.5 |
| Stride jump | 2.1 |
| Lateral step-ups (15cm) | 2.1 |
| Forward step-ups (15cm) | 2.2 |
| Running | 2.6 |
| Dance step | 2.7 |
| Step-up (30cm) | 2.7 |
| Lateral step-up (30cm) | 3.1 |
| Single leg forward leap | 3.1 |
| Hopping on single leg | 3.4 |
| Jump take off | 3.5 |
| Heel drop | 3.6 |
| Jump squat | 3.8 |
| Side-to-side jumps | 3.9 |
| Star jump | 4.3 |
| Foot stomp | 4.6 |
| Vertical jump | 4.7 |
| Tuck jump | 4.8 |
| Side-to-side jump over rope | 5.1 |
| Depth jump (30cm) | 5.2 |
| Drop jump (30cm) | 5.5 |
| Forward/backward squat jump | 6.3 |
| Vertical squat jump | 7.1 |
Despite the potential benefits of weight-bearing activities on bone, additional studies are needed to determine the safety, efficacy and feasibility of this mode of training for postmenopausal and older women at varying levels of fracture risk, and whether there is a dose-response relationship. To reduce the risk of injury, it is recommended that sedentary people or those with any functional impairments undertake a period of lower limb muscle strengthening and core stability training prior to attempting weight-bearing impact exercises. For people with severe osteoporosis, a recent history of fracture or other comorbidities such as pain from osteoarthritis, weight-bearing impact exercise may be contraindicated.18 However, a diagnosis of osteoarthritis should not preclude the prescription of weight-bearing activities; this should be based on each individual's level of pain. Indeed, the findings from a 12-month intervention in postmenopausal women with mild knee osteoarthritis provide promising results with regard to the benefits of a high impact, multidirectional exercise program on femoral neck bone mass compared to controls, with no adverse effects on the biochemical composition of knee cartilage.66 A systematic review of nine RCTs also concluded that knee joint loading exercise does not appear to be harmful for articular cartilage in people at increased risk of, or with, knee osteoarthritis.67 However, the quality of the evidence was low and thus further studies are needed to evaluate the influence of low, moderate and high impact activities in older adults with various form of (osteo)arthritis.
Multi-modal exercise trainingExercise interventions incorporating multi-modal programs that include two or more activity modes, such as weight-bearing activities, PRT and/or power training and balance/mobility training, are currently recommended for the prevention of osteoporosis and fractures because they have been shown to positively influence multiple skeletal and fall-related risk factors.18,51 For instance, a 12-month community-based RCTs in 162 older adults found that a multi-modal exercise program of traditional and high velocity PRT with multi-directional weight-bearing impact exercises and challenging balance/mobility training performed three times per week was effective for improving femoral neck and lumbar spine BMD, muscle strength, functional muscle power (timed stair climb) and dynamic balance compared to usual care controls.68 Similarly, an 8 month trial in 101 postmenopausal women with osteopenia or osteoporosis found that twice weekly, 30min high intensity resistance and impact training (four exercises, 5 sets of 5 repetitions at >80–85% of maximum muscle strength) maintained or improved hip and spine BMD as well as various functional measures relative to controls.69 These findings are consistent with the results from a meta-analysis of exercise interventions in postmenopausal women (11 RCTs including 1061 women) which found that programs integrating different exercise modalities (resistance, impact and multi-directional dynamic aerobic activities) positively affected proximal femur and lumbar spine BMD.70
There is also some preliminary evidence to support an anti-fracture effect of multi-modal exercise training. In a 16-year follow-up to a multi-modal supervised and home-based exercise intervention in which 39 postmenopausal women continued to train and 28 served as controls, exercise training was associated with a significant reduction in the risk of clinical lower trauma fractures [17 versus 11 clinical low-trauma fractures; RR 0.51 (95% CI 0.23–0.97); P=0.046] and fracture rates [24 versus 13; rate ratio 0.42 (95% CI 0.20 to 0.86); P=0.018].71 Although these findings must be interpreted with caution given the small sample size, there is also evidence that multi-modal programs are effective for preventing falls in the elderly.72 Finally, the results from a Delphi consensus process recommended multicomponent exercise training that includes resistance and balance training with emphasis on daily balance and spinal extensor muscle training and guidance of safe movements, for individuals with osteoporosis with or without vertebral fractures.73
Others modes of exercise trainingThere are few well-designed, long-term RCTs to support the benefits of other forms of exercise training on hip and/or spine BMD in postmenopausal women, including Tai Chi, Yoga and Pilates. One alternative form of mechanical loading that has been promoted to elicit a positive skeletal response is low intensity, high frequency whole body vibration training (WBV). This modality uses mechanical (vibrational) stimulation to load the body via vibrating platforms. However, at least two long-term (18–24 month) RCTs have failed to detect any beneficial effects of WBV on BMD in older adults and individuals with osteoporosis.74,75 A 2017 systematic review and meta-analysis of 15 RCTs in adults aged 50 years and over also found that WBV had no overall effect on bone outcomes, but there was evidence to support a reduction in fall rate.76 The lack of any consistent effects on bone may be related to differences in the type of vibration used (side-alternating vs oscillations), frequency, intensity or cumulative dose, body position (e.g. standing versus semi-flexed knee) and study methodology. Indeed, a stimulus focused meta-analysis reported that a cumulative dose over 1000min with side-alternating platforms at magnitudes higher than 3g (where 1g=earth's gravitational field) and/or with a frequency lower than 25Hz were associated with positive skeletal responses in older women.77 Despite these findings, some concerns have been raised around the safety of high-intensity vibrating platforms (e.g. increased falls risk, disorientation, musculoskeletal problems including low back pain).78 Thus, it is currently premature to recommend WBV as a safe and efficacious form of training to prevent osteoporosis.
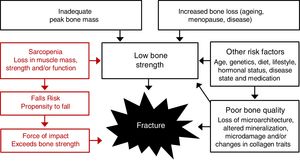
Effects of exercise training on bone strength and its determinants in postmenopausal womenA clinically important question that remains unanswered is whether training-induced improvements in areal BMD are associated with improved or maintained whole bone strength, particularly at common fracture sites. Although areal BMD (g/cm2) is an important contributor to bone strength, the ability of bone to resist fracture is also dependent on a number of other interrelated factors, including the size, geometry, microarchitecture and intrinsic material properties of bone (porosity, matrix mineralization, collagen traits, microdamage). To date, the findings from a limited number of long-term (≥12-month) exercise intervention trials in postmenopausal and older women using three-dimensional imaging tools [computed tomography (CT) or peripheral computed tomography (pQCT)] to quantify changes in bone strength, geometry and cortical and/or trabecular volumetric BMD (vBMD) have been equivocal.79–83
There is some evidence to support an exercise-induced maintenance (or increase) in cortical area or thickness at the tibia due to a reduction in the rate of endocortical bone loss (or endocortical bone formation),82 but no exercise trials have reported periosteal bone apposition in postmenopausal women. This is important because small changes in bone size (periosteal apposition) can lead to greater improvements in bone strength, with or without changes in BMD, because the resistance of bone to bending and torsional forces is related exponentially to the fourth power of its diameter. However, given the normal changes that occur on the periosteal surface throughout adult life are reportedly very small (2–5μm/year),84 further long-term studies are needed using high resolution imaging techniques [e.g. high resolution (HR)-pQCT] that have the capability to detect any subtle improvements that may occur on this surface in response to exercise.
Another important unanswered question is whether exercise training can alter bone material properties (e.g. collagen, mineralization, microdamage). At present, it is difficult to quantify changes in the material properties of bone in humans, but microindentation (a novel method for measuring the resistance of cortical bone to indentation) has emerged as a promising tool for the assessment of tissue-level material properties of cortical bone. In a 3-month unilateral progressive jumping intervention in postmenopausal women using a reference probe indentation (RPI) device, Sundh et al.85 reported significant 7% tibial gains in RPI-derived bone material strength index (BMSi) in the intervention compared to control leg, independent of any changes in bone geometry, microarchitecture or vBMD. Although these findings must be interpreted with caution due to the short-term follow-up period, this study provides some evidence that exercise may improve bone quality. In part support of these findings, a meta-analysis of six exercise RCTs in postmenopausal women reported that lower extremity exercises led to a modest (∼0.9%) but significant improvement in tibial shaft cortical vBMD (as well as distal tibia trabecular vBMD).86 Since changes in cortical vBMD are reported to reflect changes in the porosity and/or the mineralization of bone, these findings provide some indirect evidence that exercise may improve bone material properties and bone strength. Whether this is related to a reduction in intracortical porosity, increased bone mineralization or a combination of these factors cannot be determined and warrants further investigation.
RecommendationsDetailed exercise recommendations in terms of the type(s) of exercises and the frequency, intensity and dose of training that should be prescribed for the prevention of osteoporosis are outlined in Table 2. Any exercise prescription designed to optimize musculoskeletal health and function must be tailored to each individual's needs and preferences to optimize adherence, and consider the five key training principles (specificity, progressive overload, reversibility, initial values and diminished returns). For individuals at moderate to high risk of fracture due to osteoporosis and/or with functional limitations, it is advisable that a physical therapist or accredited exercise physiologist undertake a comprehensive pre-exercise evaluation and prescribe an individualized exercise program that includes fall prevention and spine sparing activities to reduce the risk of vertebral fractures.
| Type | Frequency | Intensity | Dose | Exercises/precautions |
|---|---|---|---|---|
| Progressive resistance training | ≥2 days per week | Start with slow and controlled movements and emphasize correct lifting technique. Progress to 75–85% of 1-RM (5–7/8 on Borg 0–10 point RPE scale or hard-very hard). Consider progressing to high velocity (power) resistance and functional training for lower extremities to increase rate of loading and improve movement speed and power. Light-to-moderate loads (30–70% 1-RM) can be used. | ▪ ≥8 exercises targeting muscles attached too or crossing the hip and spine ▪ At least 2 sets ▪ 8–12 repetitions ▪ 1–3min rest between sets | Exercises: squats, lunges, hip abduction/adduction, leg press, thoracic/lumbar extension, plantar/dorsi-flexion, abdominal/postural exercises, bent over row, wall/counter/floor push up, triceps dips and lateral shoulder raises. ▪ Emphasize exercises performed in a standing (weight-bearing) position. ▪ Use caution with lifting weights higher than shoulder height to limit rotator cuff injury. ▪ For individuals with low spine BMD avoid spine flexion or twisting and encourage spine-sparing strategies. ▪ Include core stability and postural strengthening/endurance exercises as well as pelvic floor activities. |
| Weight-bearing impact exercise | 4–7 times per week | Moderate to high impact activities (>2–4 BW), as tolerated. Increase height of jumps, step height, weights or a weighted vest and incorporate change of direction movements. For sedentary individual and those with poor muscle strength or function, start with PRT for 6–12 weeks to strengthen lower limb muscles and/or introduce low impact exercises and core muscle training. | ▪ 50–100 jumps per session divided into 3–5 sets of 10–20 repetitions. ▪ 1–2min rest between sets. | Multidirectional and novel loading activities: jumping, bounding, skipping, hopping, bench stepping and drop jumps or participation in weight-bearing sports (e.g., tennis, dancing, netball, recreational gymnastics and football). ▪ Teach correct landing technique. ▪ Progress slowly. ▪ Intersperse between strength and balance exercises. ▪ For those with incontinence issues first strengthen pelvic floor muscles and avoid jumping exercises with feet wide apart. ▪For those with (osteo)arthritis, prescribe within limits of pain. |
| Challenging balance, stepping and mobility | Accumulate at least 2–3h per week. This could be achieved within other exercise bouts during the course of a week. | Must be progressively challenging (close to limit of balance) and preferably specific to everyday functional tasks. Progress to dynamic/mobility and rapid stepping exercises and introduce secondary motor or cognitive tasks to improve dual task performance. | Incorporate into daily activities or combine with resistance or impact exercise (e.g., balance for 10–30s while waiting for kettle to boil, cooking or watching TV). | Include static and dynamic movements: reduce base of support, shift weight to limits of stability (e.g., leaning/reaching), perturb centre of mass, stepping over obstacles, alter surface (foam mats) and multi-sensory activities (e.g. reduce vision) and dual tasking. Consider Tai Chi and rapid stepping movements in different directions. For individuals with impaired balance or high fracture risk, start with static and progress to dynamic balance exercises. |
BW, body weight; RPE, Rating of Perceived Exertion; 1-RM, one-repetition maximum.
In accordance with most national physical activity guidelines, women should accumulate ≥150min per week of moderate to vigorous intensity physical activity. To realistically accomplish all of the above therapeutic goals, one could combine activities e.g., lunges as a leg strengthening exercise that also challenges balance, step class that includes impact exercise and moderate/vigorous aerobic challenge and simultaneously challenges balance.
Exercise training for postmenopausal women is an effective approach to improve multiple fracture risk factors, but the benefits are dependent on the type and dose prescribed. At present, the optimal training program to prevent osteoporosis and related fractures has not been determined, but there is a growing body of evidence supporting the role of multimodal programs that incorporate short bouts of novel or diverse weight-bearing impact loading activities, progressive resistance exercises targeting muscles attached to or crossing the hip and spine, and functionally challenging balance and mobility activities. Despite these guidelines, further dose-response studies in humans are needed to refine the osteogenic loading characteristics and to quantify the minimum (or optimal) dose of exercise required to improve or preserve skeletal integrity and prevent fragility fractures. To gain a greater insight into the magnitude and distribution of bone strains within the proximal femur and spine, and the specific muscles contributing to such strains, further studies should apply advanced musculoskeletal modelling approaches with three-dimensional imaging techniques in a range of cohorts at varying fracture risk.
Conflicts of interestThe authors declare no conflicts of interest.
This paper is part of a Special Issue on Women's Health Physical Therapy.