Excessive gestational weight gain is associated with several adverse events and pathologies during pregnancy.
ObjectiveThe purpose of this study was to examine the effects of an exercise program throughout pregnancy on maternal weight gain and prevalence of gestational diabetes.
MethodA randomized controlled trial was designed that included an exercise intervention group (EG) and standard care control group (CG). The exercise intervention included moderate aerobic exercise performed three days per week (50–55minutes per session) for 8–10 weeks to 38–39 weeks gestation.
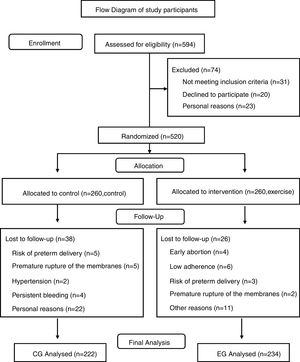
Results594 pregnant women were assessed for eligibility and 456 were included (EG n=234; CG n=222). The results showed a higher percentage of pregnant women gained excessive weight in the CG than in the EG (30.2% vs 20.5% respectively; odds ratio, 0.597; 95% confidence interval, 0.389–0.916; p=0.018). Similarly, the prevalence of gestational diabetes was significantly higher in the CG than the EG (6.8% vs 2.6% respectively; odds ratio, 0.363; 95% confidence interval, 0.138–0.953; p=0.033).
ConclusionThe results of this trial indicate that exercise throughout pregnancy can reduce the risk of excessive maternal weight gain and gestational diabetes.
Pregnancy and delivery are biological processes that can have a significant impact on maternal health and newborn wellbeing. Research has shown that events that occur during pregnancy may influence both maternal and fetal future health outcomes.1,2
The impact that gestational weight gain can have on health outcomes has been especially recognized by health care professionals as a potential factor that may influence maternal and fetal wellbeing. Excessive gestational weight gain is associated with several adverse events and pathologies. Many studies report complications related to the wellbeing of the mother, fetus and even the newborn and infant due to inappropriate maternal weight gain during pregnancy.3–8
Gestational diabetes mellitus (GDM) is defined as “carbohydrate intolerance with onset or first recognition during pregnancy”9 and it is among many problems that are highly related to excessive maternal weight gain.10 Indeed the prevalence of GDM is increasing in parallel with overweight and obesity in the obstetric population.11,12 Current trends for weight gain among women of reproductive age are alarming.13,14
Precise estimates of GDM prevalence are not clear. A recent meta-analysis reported that the prevalence of GDM in Europe is 5.4%.15 According to the American Diabetes Association (ADA), GDM complicates approximately 7% of all pregnancies.16 Regardless of the variability presented in available studies, data from western countries suggests that the prevalence of GDM is increasing.17–19 Women diagnosed with GDM have a higher risk for future diabetes, with approximately 50% of women developing type 2 diabetes within 5 years of delivery.20
Many studies support the association of GDM with several adverse maternal and fetal outcomes.21–23 Additionally, there are some data that suggest an increase in fetal malformation and perinatal mortality.24–26
Although research supports that healthy lifestyle modifications may have a positive impact on metabolic factors among overweight and obese pregnant women, evidence for specific effective approaches to prevent GDM are needed.27 Research to identify modifiable factors that might help prevent excessive maternal weight gain and abnormal glucose tolerance or GDM, in the pregnant population is needed and has urgent public health importance.28,29 One such modifiable factor may be exercise performed during pregnancy.
The existing literature suggests that physical activity before and during pregnancy may be an effective public health and clinical strategy for GDM prevention and treatment.30 This effect might be explained by the widely accepted influence that physical activity has on preventing weight gain.31
Research has supported exercise during pregnancy as an effective intervention to prevent excessive gestational weight gain.32 Furthermore, exercise during pregnancy has been identified as an effective approach to control blood sugars to help prevent and manage GDM.33 Previous studies carried out with pregnant women however have conducted physical activity programs using small sample sizes and/or lacking supervision.34,35
The main aim of this randomized controlled trial (RCT) was to examine the influence of a supervised exercise program throughout pregnancy on maternal weight gain and incidence of GDM. As a secondary objective, the effect of the exercise program on other maternal and neonatal outcomes was also examined. We hypothesized that maternal physical exercise would be associated with a reduction of both excessive maternal weight gain and prevalence of GDM without adverse effects on other maternal and newborn outcomes.
MethodsThe present RCT (clinical trial registration number NCT02109588) was conducted between March 2014 and January 2017 following the ethical guidelines of the Declaration of Helsinki, last modified in 2000. The research protocol was reviewed and approved by the Hospital Severo Ochoa (Madrid, Spain) ethics review board (240-09). Participants enrollment began in April 2014.
Participants and randomizationA total of 594 Spanish-speaking (Caucasian) healthy pregnant women from two primary care medical centers (Centro de Salud Los Pedroches, Centro de Salud Leganés Norte, Madrid, Spain) were recruited during their first prenatal visit (Fig. 1). They were informed about the nature of the study and assessed for eligibility. Women with singleton and uncomplicated pregnancies (no type 1, 2 or gestational diabetes at baseline), with no history or risk of preterm delivery (i.e. ≥1 previous preterm delivery) and not participating in any other trial were invited to participate. Women not planning to give birth in the same obstetric hospital, or with no medical follow-up throughout pregnancy were not included in the study. Women having any serious medical conditions (contraindications) that prevented them from exercising safely were also not included.36
A computer-generated list of random numbers was used to allocate the participants into the study groups following other previous studies. Allocation ratio was 1:1. The randomization blinding process (sequence generation, allocation concealment and implementation) was performed by three different researchers. The treatment allocation system was set up so that the researcher who was in charge of randomly assigning participants to each group did not know in advance which treatment the next person would receive (i.e. concealed allocation).
Women who were randomly allocated to the Exercise Group (EG) received similar standard care and performed an exercise program throughout pregnancy. Women randomly allocated to the Control Group (CG) received obstetric standard care from health professionals. Women were excluded if they did not conform to the specifications of the allotted group. All the participants signed an informed consent.
Exercise intervention37,38Pregnant women in the intervention group received standard care and all aspects of a structured and supervised moderate exercise intervention program three days per week (55–60min per session) from the 8–10th week of pregnancy (immediately after the first prenatal ultrasound) to the end of the third trimester (weeks 38–39). The exercise protocol was supervised by a qualified of physical activity and sport science professional (ten years of experience). A total of 83–85 group training sessions were originally planned for each participant in the event of no preterm delivery. The exercise program met the standards of the American College of Obstetricians and Gynecologists36 and included the following seven sections:
- i.
Gradual warm-up
- ii.
Aerobic exercises
- iii.
Light muscle strengthening
- iv.
Coordination and balance exercises
- v.
Stretching exercises
- vi.
Pelvic floor strengthening
- vii.
Relaxation and final talk
Women used a heart rate (HR) monitor (Accurex Plus, Finland) during the training sessions (HR was consistently under 70% of age-predicted maximum) and the rating of perceived exertion scale ranged from 12 to 14 (Somewhat Hard).39
The exercise session started with a light-intensity, 10-min warm-up consisting of walking and static stretching (avoiding muscle pain) of most muscle groups (upper and lower limbs, neck and trunk muscles). Similarly, the exercise session finished with a light-intensity, 10-min cool-down including the same exercises as the warm-up period plus relaxation and pelvic floor muscle training. As a motivational strategy, a final talk was done to promote extensive counseling and provide information to ensure that the participants received clear instructions on how to have an active pregnancy and emphasizing the importance of regular (not occasional) exercise throughout pregnancy.
The main section of the exercise session after the warm-up was 30–35min in length and included moderate-intensity aerobic exercises and resistance exercises. Aerobic exercises consisted of low-impact aerobic dance, involving the upper and lower limbs. Aerobic dance bouts were approximately 3–4min long and included stretching and relaxation followed by a one minute break.
Light muscle strengthening was also included in each session. Strengthening exercises engaged major muscle groups (pectoral, back, shoulder, upper and lower limb muscles) to promote good posture, prevent low back pain and strengthen the muscles used in labor and the pelvic floor (third trimester). Exercises were performed using the full range of motion and involved barbells (3kg/exercise) and low-medium-resistance elastic bands (Therabands). The exercises included biceps curls, arm extensions, arm side lifts, shoulder elevations, bench presses, seated lateral row, lateral leg elevations, leg circles, knee extensions, knee (hamstring) curls, ankle flexions and extensions. Exercises involving extreme stretching and joint over-extension, ballistic movements or jumps were avoided, and exercises in the supine position on the floor were not performed for more than 2min.
As pregnancy progresses, women may experience difficulty with balance therefore all coordination and balance exercises consisted of easy activities using sport equipment (foam balls, cords, etc.) for support.
To maximize program safety, adherence and efficacy, all sessions were: (i) supervised by a qualified fitness specialist (ten years of experience) and with an obstetrician's assistance; (ii) accompanied by music; and (iii) performed in the Health Care Center in a spacious, well-lit room under favorable environmental conditions (altitude 600m; temperature 19–21°C; humidity 50–60%). An adequate intake of calories and nutrients was confirmed before the start of each exercise session.
The intervention involved group sessions of 12–15 participants.
Adherence to the training program was ≥80% in the intervention group that was measured by a qualified fitness specialist using a checklist of attendance for each session.
Standard-care (CG)The women assigned to the standard care CG attended regular scheduled visits to their obstetricians and midwives (according to Hospital protocol), usually every 4–5 weeks until the 36–38th week of gestation and then weekly until delivery. They received general nutrition and physical activity counseling from their health-care provider.
Women were not discouraged from exercising during pregnancy on their own. However, similar to our previous studies women in the CG were asked about exercise habits once each trimester using a “Decision Algorithm” (by telephone).37
Participant demographicsInformation about demographics, including pre-pregnancy Body Mass Index (BMI), parity, educational level, previous physical activity habits, smoking status, previous pre-term birth and previous miscarriage was obtained at the first prenatal visit either by reviewing the medical records or by a telephone interview. The inclusion/exclusion criteria was determined at this initial visit by the attending obstetrician.
OutcomesPrimary outcomesTotal maternal weight gain (kg) and excessive gestational weight gain (yes/no) were recorded. Total gestational weight gain was calculated on the basis of the pregravid weight (first prenatal consult) and weight at the last clinic visit before delivery (week 36–38). Excessive gestational weight gain was defined according to the recommendations of the 2009 Institute of Medicine (IOM) guidelines40 categorized by pre-pregnancy BMI for each woman: >18kg for underweight; >16kg for normal weight; >11.5kg for overweight; and >9kg for obese women. Cases of gestational diabetes and 1h Oral Glucose Tolerance Test (OGTT) information was collected from hospital records (week 24–26).
Secondary outcomesMaternal gestational age at delivery, type of delivery and birth weight were collected from hospital records. Newborns were classified as having macrosomia when birth weight was >4000×g and low birth weight was defined as <2500×g.41 Primary and secondary outcomes were assessed by healthcare professionals.
Statistical analysesSample size was determined based on a priori widely accepted power calculation.42 In total, 340 subjects were needed to achieve 80% power to detect a statistically significant difference in maternal weight gain taking into account previous data on this variable. The sample size was intentionally increased to account for patient withdrawal and possible problems for follow-up.
A Kolmogorov–Smirnov test was performed to verify the normality of the data in the study variables and showed that it was non-parametric (p<0.05). Thus, Mann–Whitney tests were performed to analyze possible differences between the groups for continuous variables (maternal weight gain, oral glucose tolerance test (OGTT), maternal age, gestational age, pre-pregnancy BMI and birthweight). The Pearson χ2 test was completed with the observation of standardized adjusted residuals and was used to assess differences between categorical variables (excessive weight gain, gestational diabetes, parity, mode of delivery). Statistical tests used a 2-sided 0.05 alpha level and SPSS 24.0 was used to analyze the data. All analyses were done on an intention-to-treat basis.
Results (Fig. 1)Baseline characteristicsBaseline characteristics for both groups are listed in Table 1 and were similar between groups for most of the variables.
Maternal characteristics.
| CG (n=222) | EG (n=234) | |
|---|---|---|
| Maternal age*(mean±SD) | 31.04±3.78 | 31.75±4.68 |
| Pre-pregnancy BMI (mean±SD) | 23.66±3.81 | 23.50±3.79 |
| Pre-pregnancy BMI categories (n/%) | ||
| <18 | 6 (2.7%) | 5 (2.1%) |
| 18–24.9 | 157 (70.7%) | 160 (68.4%) |
| 25–29.9 | 45 (20.3%) | 54 (23.1%) |
| >30 | 14 (6.3%) | 15 (6.4%) |
| Parity (n/%) | ||
| No previous birth | 162 (73%) | 142 (60.7%) |
| One previous birth | 54 (24.3%) | 77 (32.9%) |
| More than one previous birth | 6 (2.7%) | 15 (6.4%) |
| Previous miscarriage (n/%) | ||
| None | 162 (73%) | 173 (73.9%) |
| One | 53 (23.9%) | 51 (21.8%) |
| Two or more | 7 (3.2%) | 10 (4.3%) |
| Study levels (n/%) | ||
| Primary school | 76 (34.2%) | 30 (12.3%) |
| Secondary school | 97 (43.7%) | 87 (37.4%) |
| Tertiary education | 49 (22.1%) | 117 (50.0%) |
| Smoking (n/%) | 49 (22.1%) | 44 (18.8%) |
Differences in main outcomes (maternal weight gain, OGTT and cases of GDM) are presented in Table 2. Maternal weight gain was significantly lower in the EG compared to the CG (12.19 vs 13.33kg respectively, U=22044, p=0.005). In line with these results, standardized adjusted residuals in Pearson χ2 suggested that the ratio of women that gained excessively was higher in the CG than the EG (30.2% vs 20.5% respectively; odds ratio, 0.597; 95% confidence interval, 0.389–0.916; p=0.018). A significant difference was also found for the OGTT results (EG=116.56 vs CG=121.63mg/dL, U=23,158, p=0.045). Finally, standardized adjusted residuals in Pearson χ2 suggested that the ratio of women diagnosed with GDM was higher in the CG than the EG (6.8% vs 2.6% respectively; odds ratio, 0.363; 95% confidence interval, 0.138–0.953; p=0.033).
Maternal weight gain, oral glucose tolerance test and gestational diabetes.
| CG (n=222) | EG (n=234) | P value | Between group differences | 95% CI | |
|---|---|---|---|---|---|
| Maternal weight gain* (mean±SD) | 13.33±4.08 | 12.19±3.70 | .005 | 1.14±0.37 | 0.42–1.86 |
| Maternal excessive weight gain (n/%) | 67 (30.2%) | 48 (20.5%) | .018 | ||
| OGTT** (mean±SD) | 121.63±29.56 | 116.56±29.69 | .045 | 5.43±2.70 | 0.12–10.74 |
| Gestational diabetes (n/%) | 15 (6.8%) | 6 (2.6%) | .033 |
Other outcomes of interest analyzed in the study are presented in Table 3. Among maternal outcomes, no differences were found for gestational age, number of preterm deliveries or mode of delivery. In regards to newborn outcomes, no differences were found for birthweight between study groups. Our results showed that, although the χ2 test was not significant, the ratio of neonate macrosomia was slightly higher in the CG than in the EG (7.2% vs 3.4% respectively; odds ratio, 0.456; 95% confidence interval, 0.191–1.087).
Other maternal and newborn outcomes.
| CG (n=222) | EG (n=±234) | P value | Between group differences | 95% CI | |
|---|---|---|---|---|---|
| Mother | |||||
| Gestational age* (mean±SD) | 277.18±9.75 | 277.21±12.81 | .45 | −0.04±1.07 | −2.14 to 2.07 |
| Preterm delivery (>37 weeks) (n/%) | 7 (3.2%) | 10 (4.3%) | .53 | ||
| Mode of delivery (n/%) | |||||
| Normal | 138 (62.2%) | 156 (66.7%) | .41 | ||
| Instrumental | 38 (17.1%) | 30 (12.8%) | |||
| Cesarean | 46 (20.7%) | 48 (20.5%) | |||
| Newborn | |||||
| Birthweight** (mean±SD) | 3256.34±465.94 | 3266.58±451.52 | .60 | −10.23±43.00 | −94.74 to 74.28 |
| Macrosomia (n/%) | 16 (7.2%) | 8 (3.4%) | .07 | ||
The aim of the present study was to examine whether regular and supervised physical exercise during pregnancy can influence prevention of excessive maternal weight gain, and GDM, which are both closely related factors. Similar to our previous work, the main strength of the current study is the combination of light resistance, toning, aerobic dance, coordination, stretching and pelvic floor muscle training in the same program throughout pregnancy and examining the resultant effects on outcomes. The main finding of this study is that the exercise program reduced the total (mean) maternal weight gain as well as the cases of excessive weight gain and GDM.
Our results are relevant from a clinical and health care point of view due to the increasing prevalence of these two parameters in recent years, in parallel with the alarming rise of worldwide overweight and obesity.11,12 Furthermore the interpretation of our results promote the use of moderate and supervised physical exercise throughout pregnancy as a method to increase prevention of pregnancy complications and improve quality of life for pregnant women without adverse effects on maternal and fetal well-being.
Regarding the external validity and generalizability of our findings the high adherence (≥80% attendance) of this large RCT for all pre-pregnancy BMI categories strongly supports the extension of the present results to the healthy pregnant population.
In regards to the newborn health outcomes, although birth weight was similar in neonates between the CG and the EG, the percentage of newborns with macrosomia was lower in the EG. We had previously observed37,38 this effect, and therefore this study provides additional evidence that physical exercise may improve perinatal outcomes by preventing excessive accumulation of weight during fetal development.
Other authors have previously investigated the impact of prenatal exercise on excessive gestational weight gain and GDM.43–55 Among the great variety of study designs used, RCTs are the most reliable as they allow management of independent variables (exercise program design). Current literature available on RCTs includes a great variety of exercise programs used. It might explain the difficulty in determining the exact type and frequency of exercise during pregnancy that is required to prevent and treat GDM.
From a methodological point of view the more adaptive/desirable outcomes are reported by those studies in which a supervised intervention (exercise program) including a large variety of exercises (aerobic, resistance, pelvic floor and muscle strengthening, stretching, etc.) have been provided throughout the pregnancy.46–51
Regardless of the variability among exercise interventions, most researchers agree that prenatal exercise is an excellent way for controlling maternal weight gain during pregnancy. Our results are in consensus with many authors,43–46 and with our previous studies on this health outcome.47,48
However, as we mentioned previously the relationship between exercise and GDM has been unclear. While some evidence suggests a high efficacy in the use of exercise as a preventive method,49–51 literature has been inconsistent on the effect of prenatal exercise when used as a treatment method for reducing risk factors for GDM.42–55 Differences in exercise programs may explain this. In our opinion the variance in the duration of the programs, length of the sessions, adherence and especially the type of exercises used, contribute to the differences observed in the results of studies.
Strengths and limitationsThe major strengths of our study include the large number of participants in this RCT, the high adherence to intervention (>80% attendance) and the identification of those women in the CG who did not remain sedentary. In our opinion, the present results provide healthcare practitioners with evidence-based information that can be used to recommend supervised physical exercise throughout pregnancy to maintain or improve the quality of life of pregnant women including labor and birth.
One limitation of the current study was that nutrition or energy intake was not assessed, however, all pregnant women had (by their obstetricians and midwives) standard care which included regular information about a healthy lifestyle during pregnancy including nutrition information. Therefore the supervised exercise program was the only difference between study groups. In addition, we found differences between the study groups for parity and educational level of participants which could potentially influence the results.
The impracticality of instituting this type of a supervised activity program for pregnant women on a mass scale may be another potential limitation of the present study. Furthermore, our study focused on a Spanish population and was conducted in two tertiary care hospitals in Madrid, which may lower the external validity of our findings.
ConclusionWe conclude that a supervised physical exercise program initiated early and maintained throughout pregnancy can reduce the risk of excessive maternal weight gain and GDM.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to acknowledge the technical assistance of the Gynecology and Obstetrics Department of “Hospital Severo Ochoa” and the health practitioners of Centro de Salud Los Pedroches, Centro de Salud Leganés Norte, Madrid, Spain.
The authors also would like to acknowledge the technical assistance for the English revision to Taniya Singh Nagpal from University of Western Ontario (Canada).
This paper is part of a Special Issue on Women's Health Physical Therapy.
Trial Identifier: NCT02109588. https://clinicaltrials.gov/ct2/show/NCT02109588