Multiple sclerosis has a great disability burden. Management of the disease is complex, and patients often seek new conservative approaches.
ObjectiveTo investigate the effect of low-frequency pulsed electromagnetic field (PEMF) therapy, compared to placebo, on the level of fatigue, walking performance, symptoms of depression, and quality of life (QOL) in patients with relapsing-remitting multiple sclerosis (RRMS).
MethodsForty-four adults with RRMS and minimal to significant disability were randomly assigned to a 4-week protocol using a PEMF or a placebo whole-body mat. The PEMF group were initially treated with 15Hz frequency, gradually increased to 30Hz (intensity between 25-35µT). The primary outcome was fatigue, assessed with the Fatigue Severity Scale (FSS) and the Modified Fatigue Impact Scale (MFIS). Secondary measures included walking function (GAITRite system and Timed 25-Foot Walk test), the Beck Depression Inventory-II, and the Multiple Sclerosis International Quality of Life Questionnaire. Data were collected at baseline, after intervention, and at 3-months post-intervention (follow-up).
ResultsThere were no differences between groups for changes in fatigue symptoms from baseline to end of intervention (mean and 95% confidence interval FSS: -0.6, 95%CI: -1.3, 0.1; MFIS: -5.4, 95% CI: -15.1, 4.4) or at follow-up (FSS: -0.6, 95% CI: -1.4, 0.2; MFIS: -2.1, 95% CI: -10.9, 6.8). Similarly, both groups did not differ for any of the secondary outcomes at post-intervention or follow-up.
ConclusionsLow-frequency PEMF therapy is no more effective than placebo to produce changes in fatigue, gait performance, severity of depression, and QOL in people with RRMS and minimal to significant disability.
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease that often presents in young adults.1 In 2020, 2.8 million people worldwide were estimated to live with MS,2 and approximately 85% of them were diagnosed with the relapsing-remitting type (RRMS).1 Disease activity, e.g., rate of relapses and severity of damage, can manifest as different symptoms and lead to restricted function, e.g., walking impairments.3 Fatigue, primary or secondary, appears in the early stages, can be present for years, and is the most frequent and severe MS-related symptom.4 Depressive disorders are a rather prevalent comorbidity in the context of fatigue4 and may interfere with independence and ability to work.5 Between 50% to 80% of people with MS, even those with mild levels of disability, report their walking performance (endurance, biomechanics, and variability) is impaired.6 All this together negatively impacts on the quality of life (QOL) and the ability to participate in everyday activities.3,4
Management of MS is complex. Medical treatment with disease-modifying therapies can reduce disability and the frequency of annual relapse rates, but it is also associated with adverse effects, e.g., infections, headache, and diarrhea.7 Hence, current approach needs to be multimodal and include lifestyle changes, psychological support, and rehabilitation interventions.7 Rehabilitation aims to decrease the impact of fatigue and enhance function and participation, with current evidence suggesting the positive effects of exercise.8 However, most commonly used treatments have shown limited or inconclusive effect,8 and people with MS tend to seek alternative therapies, despite those alternative options rarely being recommended in clinical guidelines.9
Peripheral low-frequency pulsed electromagnetic field (PEMF) therapy is a non-thermal, non-invasive technique that has become popular as adjuvant for treating musculoskeletal disorders.10–12 In people with neurological diseases, research about PEMF treatment is scant and limited to adults with MS,13 stroke,14 and Parkinson's or Alzheimer's disease15 to determine effects on fatigue, depression, functional status, and QOL. The limited data available, together with the variety of current parameters reported in the literature,13,14 and the placebo effect,16 can explain the conflicting findings reported to date. Electromagnetic field treatment has shown a potential neuroprotective role and seems to modulate the inflammatory and immune responses,14,15,17 which can help to accelerate sensorimotor and neurological recovery.14,17 Several studies have provided a molecular basis to support the clinical use of PEMF,12 although the underlying mechanisms of action are not well understood.11,12 The primary objective was to investigate the effects of PEMF therapy, compared with placebo, on the self-reported level of fatigue in people with RRMS. As a secondary objective, we explored the effects of PEMF therapy on walking performance, severity of depression, and QOL.
MethodsStudy designThis is a randomized placebo-controlled trial. The study design complied with the ethical guidelines of the Helsinki Declaration and the Consolidated Standards of Reporting Trials (CONSORT) requirements. The protocol was approved by the Research Ethics Committee of the Hospital Universitario Virgen Macarena, Sevilla, Spain (code: CP.CI-2077) and prospectively registered in the Australian and New Zealand Clinical Trials Registry (ACTRN12618000515291). All participants provided verbal and written informed consent prior to enrollment.
ParticipantsPatients with a confirmed diagnosis of RRMS, according to the 2017 revisions of the McDonald criteria,18 were recruited from the MS unit at a large public hospital in southern Spain. Participants between 18 and 65 years were included if they reported a moderate to high level of fatigue (score ≥ 4 in the Fatigue Severity Scale) and were able to walk without aid for at least 100m (score ≤ 5.5 in the Expanded Disability Status Scale, EDSS).19 The exclusion criteria were: a severe neuropsychologist-confirmed cognitive and/or psychiatric impairment; a relapse episode in the previous month or during the study protocol;20 a history of epilepsy or traumatic brain injury; prior lower limb fracture or severe trauma; changes in MS disease-modifying therapy within the previous month;20 having internal metallic devices;21 and pregnancy or breastfeeding.21 Participants were asked not to engage in any new treatment for their MS during the study period but were allowed to continue with their regular medication intake.
Randomization and blindingA staff member performed randomization using a computer-generated random numbers sequence in permuted blocks, considering a 1:1 distribution ratio. Allocation of the intervention was concealed with consecutively numbered sealed opaque envelopes. Participants, outcome assessors, and the therapist in charge of the intervention, remained blinded to treatment allocation.
Sample sizeSample size was estimated using the G*Power software, version 3.1.9.7 (Heinrich-Heine University, Düsseldorf, Germany). For an alpha level of 0.05 with 80% power, calculation was based on the observed effect of PEMF treatment, compared to placebo, on fatigue symptoms in this population.22 We considered 2 groups, 3 measurements and a mean between group difference after intervention of 8 points (SD = 12) on the Modified Fatigue Impact Scale. This generated a sample of 44 patients (including a 10% dropout rate) to complete the trial.
InterventionsInterventions were performed at the hospital facilities by a physical therapist with over 10 years of neurorehabilitation experience. All participants underwent a 4-week treatment protocol (5 sessions per week). Sessions were conducted during weekdays (morning time) and lasted 45 minutes. Patients were instructed to remain in a comfortable supine position, with knee extended, over a whole-body mat system. Two identical mats (A and B) were used (Duo Forte Renaissance®, ZES Brno, Brno, Check Republic). A mat green indicator lamp was lit during the entire session, and participants were told that they would not feel any specific sensations.21 Only one of the devices (mat B) delivered pulsed electromagnetic fields. Treatment started with an initial 15-Hz frequency that was automatically increased by 5-Hz every 10 min. up to 30-Hz, with a mean intensity between 25 to 35µT. The placebo group was exposed to a magnetically inactive field (mat A) for the same period and number of sessions. Information about the placebo mat was safeguarded by staff external to the study and disclosed when data collection was completed.
Outcome measuresThe self-reported level of fatigue was the primary outcome. Secondary outcomes included gait performance, the burden of depressive symptoms, and QOL. One researcher collected the clinical and demographic data. Two different assessors completed the evaluations at baseline (before randomization), immediately after the last treatment session, and at 3 months after intervention (follow-up).
The primary outcomes were the Fatigue Severity Scale (FSS) and the Modified Fatigue Impact Scale (MFIS). The FSS is a 9-item questionnaire with a physical focus, whereas the MFIS explores the effects of fatigue in the physical, cognitive, and psychosocial domains. Higher scores represent a greater severity of fatigue. The FSS and the MFIS are valid, precise, and show moderate to good reliability in people with MS.23 Estimates of meaningful clinical changes range from 5.6% to 27% for the FSS and from 4.6% to 24% for the MFIS.23,24
We also made a number of secondary outcome measures. Gait performance was assessed with the GAITRite® system (CIR Systems Inc., Franklin, NJ, USA), which is an 8-meter mat with motion sensors arranged in a grid-like pattern. This system is valid and highly reliable to monitor spatiotemporal gait parameters, e.g., speed, cadence, and functional ambulation performance, in patients with MS;25 and with the Timed 25-Foot Walk test (T25FW). A 20% change in the T25FW is considered to be clinically relevant.26 The average score after two consecutive walks was used for analysis.
The Beck Depression Inventory II (BDI-II) consists of 21 items to grade and monitor psychic aspects of depression. The BDI-II is a valid, rapid, and reliable tool, with good internal consistency in people with MS.27
Health-related QOL was self-reported with the Multiple Sclerosis International Quality of Life Questionnaire, which covers nine dimensions: daily life activities, psychological well-being, symptoms, relationships with friends, family and the healthcare system, sentimental and sexual life, coping, and rejection.28
Statistical analysisThe statistical processing of data was conducted with the software IBM Statistics Package for Social Science®, v.26 (IBM Corp, NY, USA). According to an intention-to-treat approach, patients’ data were analyzed in the group they were allocated. All randomized participants were included in the analysis, with the last observation carried forward method used to impute missing data. The Shapiro-Wilk test was used to assess the normal distribution of the variables. Data are reported as mean ± standard deviation, mean difference (MD) with 95% confidence interval (CI), or in absolute numbers (percentages). A linear mixed model for repeated measures was used to compare the changes in the outcome measures from baseline to end of intervention and at follow-up, with group (PEMF or placebo) as the between-subjects factor, and time (pre, end of intervention, and follow-up) as the within-subjects factor.
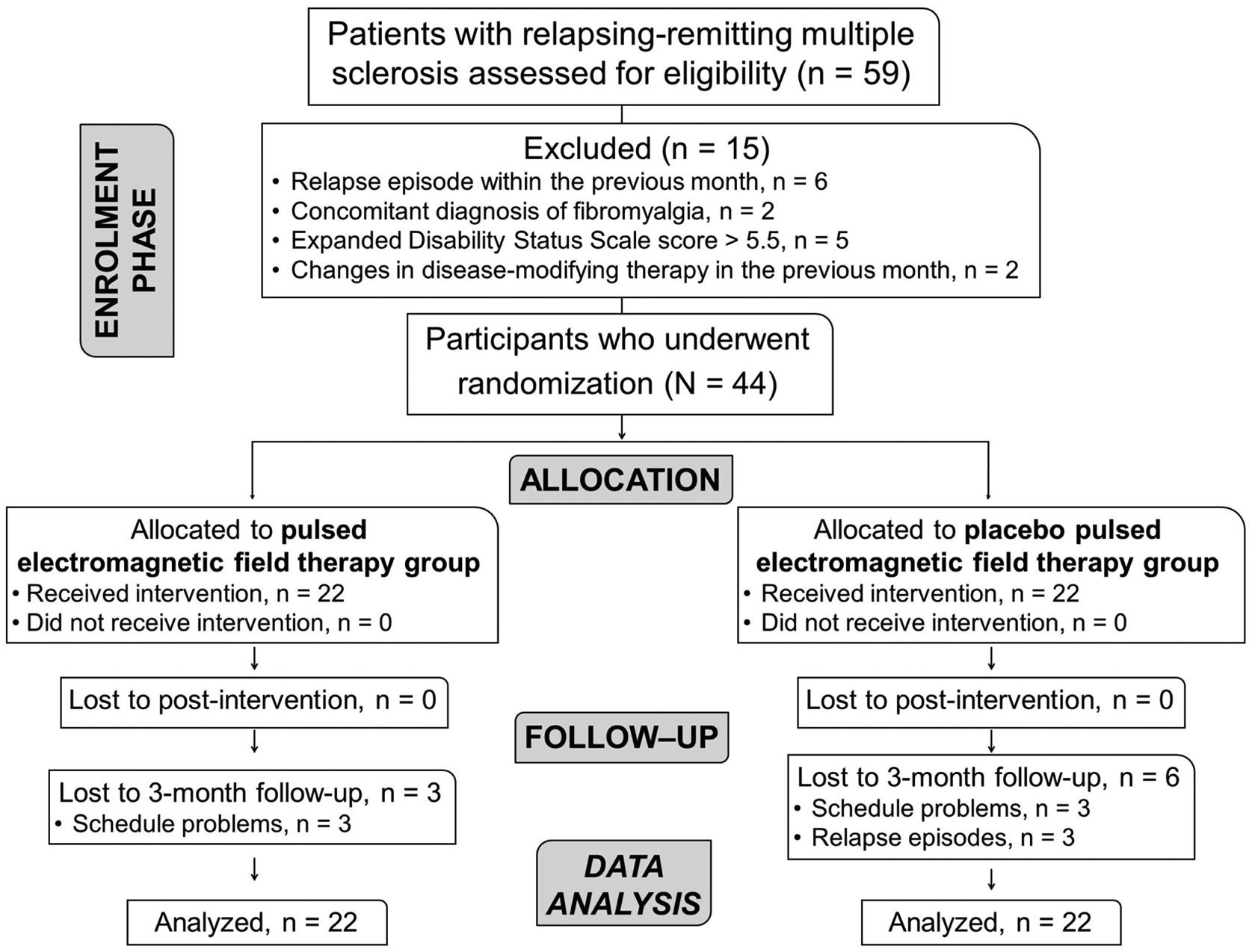
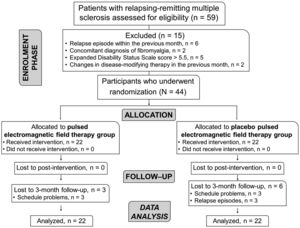
ResultsA total of 44 participants with RRMS (84.4% females), mean age of 41 ± 9.9 years, were recruited between May 2018 and September 2019. Nine patients dropped out after the post-intervention assessment (Fig. 1).
Table 1 lists the baseline demographic characteristics of the sample. All participants had a minimal to significant disability and were able to walk without aid or rest for at least 300m (EDSS score between 2 and 4.5). At baseline, the PMFT and placebo groups showed similar values for all outcomes.
Demographic characteristics of the study sample.
Data are mean ± standard deviation or frequency (proportion).
BMI, body mass index; EDSS, Expanded Disability Status Scale; PEMF, pulsed electromagnetic field.
Table 2 summarizes the scores for self-reported level of fatigue in the study groups, including the within- and between-group differences. There were no differences in changes from baseline for both fatigue measures between the PEMF group and the placebo group at the end of treatment (FSS, MD= -0.6, 95%CI: -1.3, 0.1; MFIS, MD= -5.4, 95% CI: -15.1, 4.4) or at 3 months post intervention (FSS, MD= -0.6, 95% CI: -1.4, 0.2; MFIS, MD= -2.1, 95% CI: -10.9, 6.8).
Changes in fatigue measures.
Data are mean ± standard deviation or mean difference (95% confidence interval). Bold data indicates when the 95% CI does not cross zero. PEMF, pulsed electromagnetic field.
There were no differences between groups in score changes at post-intervention or at follow-up for any of the measures of gait performance, severity of depression symptoms, and QOL (Table 3).
Changes in secondary measures (walking performance, symptoms of depression and quality of life).
Data are mean ± standard deviation or mean difference (95% confidence interval). Bold data indicates when the 95% CI does not cross zero.
QoL, Quality of life; PEMF, pulsed electromagnetic field.
The present findings demonstrated that the effect of PEMF therapy was not superior than placebo for the self-reported level of fatigue, as measured with the FSS and the MFIS. Further, PEMF therapy was also not superior to evoke changes on gait performance, depression, and QOL compared with placebo.
Regarding MS-related fatigue, between groups differences in changes did not surpass the minimum clinically important threshold (more than 2 points for the FSS and around 16 to 20 points for the MFIS).24 This is consistent with previous research on the topic. Most current evidence indicates that PEMF, either alone16,29,30 or within a multimodal rehabilitation program,31 is no better than placebo to reduce the severity of fatigue symptoms in the short,16,29–31 or medium term,16 in people with MS. In contrast, two older studies from the same research group found a positive immediate effect, although rather small, after wearing a portable PEMF device for 4 to 8 weeks.32,33 Additionally, a 3-month intervention low-frequency PEMF achieved a modest beneficial impact, compared to placebo, on the FSS and the MFIS after intervention29 and at a 3-year follow-up.22 But, the latter study had an open-label design and participants were not blinded to treatment allocation. In clinical trials, absent or questionable blinding may confound estimates of treatment efficacy.34 The magnitude of the risk of bias can depend on the intervention and the nature of the study measures.34 Placebo-controlled trials are especially important when the primary outcome is self-reported,35 and the intervention involves electrophysical modalities.36 A placebo effect has been suggested as a reason for the lack of differences between active or sham PEMF therapy. Simple rest for more than 15 minutes over a magnetically inactive PEMF mat can reduce the level of fatigue between 5% to 14%,16,29–31 which is similar to the 10% to 12% improvement observed in the MFIS in our placebo group. Although we followed previous research to establish the treatment protocol, it is questionable whether the role of placebo may be similar with shorter exposure times. This needs to be further investigated. In people with MS, physiological mechanisms of electromagnetic fields include patterns of cortical activation and inhibition,33 therapeutic effects on immune-relevant cells, and a potential increase of blood oxygen and circulation,15 although these effects may depend on the current parameters (wavelength and intensity).15 PEMF has also shown an antiinflammatory role in human and animal studies.17 However, differences in medical and fatigue-related profiles among participants exposed to the intervention may also account for the inconsistency in the scientific literature.
For the secondary outcomes, this clinical trial is, to our knowledge, the first to investigate the efficacy of PEMF on spatiotemporal gait parameters other than speed.16 Former studies have reported that a single session37 or continuous exposure to PEMF38,39 can increase walking speed and stride length, compared to placebo or other approaches, in individuals at risk of falling37,38 or with knee osteoarthritis.39 Similarly, preliminary reports indicate that PEMF therapy reduces slowness of movement, walking impairments, and freezing of gait in adults with Parkinson's disease.40,41 In contrast, and consistent with our results, de Carvalho et al.16 reported no differences on walking velocity, measured with the Timed 10 meter walk test, after an 8-week protocol (3 times a week, and 24 min per session) using active or placebo low-frequency PEMF. Based on the negative findings, the fact that PEMF is a passive and costly approach,31 and the lack of standardized protocols in terms of current parameters and exposure time,12 we cannot recommend this intervention to evoke changes in gait performance for people with RRMS. New research may help to establish more definitive conclusions.
There were no differences between groups for the BDI-II or the health-related QOL. Magnetic field therapy, delivered as repetitive transcranial magnetic stimulation, has proven to modulate cortical excitability and depressant behaviors in animal models.42 In addition, evidence-based guidelines recommend this approach to manage the burden of depressive symptoms in people with MS.43 Yet, results appear to differ when PEMF is applied peripherally at a body part or with whole-body mats. Consistent with the current results, a pilot trial demonstrated no positive impact of monopolar transmission of PEMF therapy on depression or QOL in patients with MS.30 In contrast, low-frequency PEMF was superior to placebo to evoke long-term positive changes on depression in this population.22 In women with fibromyalgia, this treatment has shown to improve health-related QOL, with no beneficial effects for depression.44 This inconclusive evidence also applies to other musculoskeletal and neurological conditions.12,45
Adverse effectsPEMF therapy is a non-invasive and purportedly safe technique,40 although little is known about the potential long-term hazards.11 As in previous research,21,32 our participants did not report acute or minor adverse events. Current recommendations suggest that magnetic field treatment should be used cautiously, with supervision, and only when recommended, because some commercially-available products may not comply with safety guidelines.46
Study limitationsWe did not include participants with severe disability or in need of a walking aid (EDSS > 4.5).19 Although this was intended to recruit an homogenous sample, it may also limit the external validity of the findings. Patients with MS show differences in inflammatory factors depending on disease activity and type, thus the antiinflammatory effect of PEMF therapy may differ at different stages of the disease. This needs to be investigated in further research that also includes a longer follow-up. Finally, the possible mediating effect of comorbidities was not investigated.
ConclusionThe study concluded that, in adults with RRMS and with minimal to significant level of disability, the use of low frequency PEMF therapy was not better than placebo to improve the level of fatigue, walking performance, severity of depression, and QOL.
Trial registeredACTRN12618000515291 (https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373996&isReview=true)
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.