In the early stages of total knee arthroplasty (TKA) rehabilitation, in which physical function in general can be affected, motor imagery (MI) might play a relevant role.
ObjectiveTo assess the impact of MI on strength, active range of motion (ROM), pain intensity, and physical function in patients with TKA.
MethodsWe conducted a systematic review and meta-analysis of randomised controlled trials. Pooled effects were calculated as standardised mean differences (SMDs) and 95% confidence intervals (CIs) for the relevant outcomes using random effects model. The certainty of evidence was assessed with GRADE approach.
ResultsThis review included 7 articles. The addition of MI to standard therapy, based on low quality of evidence, showed a moderate increase in quadriceps strength (4 studies; SMD: 0.88; 95% CI: 0.42, 1.34) and a small reduction in pain intensity (SMD: 0.63; 95% CI: 0.08, 1.19). It is unclear whether MI can provide beneficial effects for active ROM and function.
ConclusionsThere is low to very low-quality evidence that adding an MI intervention to standard rehabilitation for patients with TKA may improve quadriceps strength and pain intensity, but the effects of MI on ROM and physical function is unclear.
Knee osteoarthritis is a complex multifactorial condition that includes a range of factors such as age, sex, obesity, and family history and local mechanical factors such as previous trauma, malalignment, and general laxity.1–3 There also appears to be a genetic component involved with a number of studies indicating that genetic factors are involved in 39%–65% of cases of hand and knee arthritis amongst women.4,5 Knee osteoarthritis can be managed using a conservative or surgical approach. The conservative approach can include strength and aerobic exercises, which have shown evidence of beneficial effects on clinical outcomes,6 whereas the surgical approach can include joint arthroplasty.7
The factors that predict a good prognosis for early recovery in pain and function after joint arthroplasty include young age, regaining sensitivity, radiographic evidence of mild to moderate wear in the knee, and the absence of degenerative tearing of the meniscus.8 Total knee arthroplasty (TKA) is reserved for patients who show criteria of functional limitation or compartment degeneration greater than those mentioned, and is considered as the last option for patients with knee osteoarthritis, and is the most common procedure for these patients.5,9
People who undergo a TKA often experience reductions in quality of life and function, mainly due to loss of mobility and presence of pain, which contribute to problems in the execution of certain activities of daily life.10 There is a considerable reduction in quadriceps strength and function, which persists for several months after the surgery.11 Early rehabilitation is therefore essential to minimise the consequences of TKA.
In the early stages of TKA rehabilitation, when mobility in particular, and physical function in general, can be affected, movement representation methods might play a relevant role. One of the main movement representation methods used in rehabilitation is motor imagery (MI), which is defined as a dynamic mental process involving the internal representation of an action without its actual motor output.12 Motor imagery leads to activation of areas related to the planning, adjustment, and automation of voluntary movement in a similar form as to when the action actually occurs.13 Motor imagery has been shown to be useful in improving physical function in some clinically important outcomes. For example, previous research suggest that MI could lead to improvements of several important functional variables like mobility or strength in patients undergoing TKA.14,15
The main aim of the present systematic review and meta-analysis was to determine the effects of MI in addition to standard rehabilitation on strength, range of motion (ROM), pain intensity, and physical function in patients with TKA.
MethodsThis systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.16 The protocol of this systematic review and meta‐analysis was registered (August 2020) in an international register prior to starting the review (PROSPERO: CRD42020205353).
Search strategyThe search was performed on MEDLINE (PubMed), PEDro, Semantic Scholar, Scopus, and Google Scholar. The final search update was performed on 19 August 2020. We employed a validated search filter and adapted it to all databases.17–19 Based on international criteria, we did not limit our search to any language.20 Two independent researchers (L-MFR and PAV) conducted the search using the same methodology. Consensus was used to resolve possible disagreements between the researchers. In addition, we manually searched journals that typically publish on this topic to include all available studies. All eligible studies had their introduction, discussion, and reference sections checked for relevant studies missed by our search. We employed Mendeley reference management software (Mendeley desktop v1.17.4, Elsevier, New York, NY, USA) to remove duplicate studies.21
Eligibility criteriaThe selection criteria employed in this systematic review and meta-analysis were based on methodological and clinical factors, such as the Population, Intervention, Control, Outcomes, and Study design described by Stone.22 Studies were considered eligible for this review if they were randomised controlled trials; included participants from both sex and who were older than 18 years of age who underwent TKA surgery (primary unilateral joint arthroplasty); investigated the efficacy of MI as an independent intervention or in combination with other interventions compared to usual care or standard rehabilitation (i.e. physical therapy, exercise intervention) or with placebo interventions; and considered quadriceps strength, active ROM, pain intensity, and physical function assessed through the Time Up and Go test (TUG) as outcome measures. For the MI intervention, studies on both visual and kinaesthetic strategies and both perspectives of movement representations were considered eligible (first-person or third person). Kinaesthetic MI is performed by constructing an image of movement and, in turn, incorporates the ability to feel what is being imagined. Visual MI only constructs the image of movement. Perspective refers to whether the person sees him/herself constructing the mental image (first-person perspective) or whether he/she observes him/herself from the outside, as if reflected in a mirror (third-person perspective).
Selection criteria and data extractionTwo independent reviewers (L-MFR and PAV) screened the titles and the abstracts. Full-text articles were retrieved for all potential eligible studies. Two review authors then independently examined the full-text reports to determine whether studies met the selection criteria. Review authors resolved disagreements regarding the inclusion criteria through discussion with a third reviewer (FCM).23 Data described in the results were extracted with a standardised data extraction form that ensured that the most relevant information was obtained from each study.24
Risk of biasTwo authors independently evaluated the risk of bias of each included study, using the Cochrane risk of bias tool (version 5.1.0). This tool has seven domains: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other potential sources of bias. Each domain was scored as "yes", "no", and "unclear" and classified into one of three categories as "high risk of bias", "low risk of bias", or "unclear".
Two independent reviewers (L-MFR and MRP) assessed the risk of bias of included studies. Disagreements between the reviewers were resolved after consulting a third reviewer. The concordance between the results (inter-rater reliability) was performed using Cohen's kappa coefficient (κ): κ > 0.7 indicates a high level of agreement between assessors; κ = 0.5–0.7 indicates a moderate level of agreement; and κ < 0.5 indicates a low level of agreement.25
Certainty of evidenceThe Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework was used to assess the certainty of evidence. The GRADE is based on 5 domains: study design; imprecision; indirectness; inconsistency; and publication bias.26 The assessment of the 5 domains was conducted according to GRADE criteria and performed by two independent reviewers.27,28 Evidence was categorised into the following 4 levels accordingly: (a) High quality. Further research is very unlikely to change our confidence in the estimate of effect. All 5 domains do not have serious concerns; (b) Moderate quality. Further research is likely to have an important impact on our confidence in the estimate of effect and might change the estimate of effect. One of the 5 domains has serious concerns; (c) Low quality. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Two of the 5 domains have serious concerns; and (d) Very low quality. Any estimate of effect is very uncertain. Three or more of the 5 domains have serious concerns.27,28
Regarding the study design domain, we downgraded by one level in case there was an uncertain or high risk of bias and serious limitations in the estimate of the effect (i.e. more that 25% of the participants were from studies with low methodological quality). For inconsistency, we downgraded by one level when the I2 was greater than 50%. For indirectness, we downgraded when severe differences in interventions, study populations or outcomes were found (i.e., in absence of direct comparisons between the interventions of interest, when there were no key outcomes or only intermediate outcomes, and if more than 50% of the participants were outside the target group). In relation to imprecision domain, we downgraded by one level if there were n<300 participants for continuous data.
Data synthesis and analysisWe used MetaXL software for the quantitative analysis,29 using the same three inclusion criteria for the meta-analysis: 1) the results showed detailed information regarding the comparative statistical data of the exposure factors, therapeutic interventions, and treatment responses; 2) the intervention was compared with a similar control group (e.g., usual care or standard rehabilitation); and 3) data on the analysed variables were represented in at least 3 studies.
The inverse variance method and random effects model were used in all meta-analyses. We evaluated the statistical heterogeneity using the chi-squared test (with statistical significance set at p<0.10) and the inconsistency index (I2).30 An I2 between 0% and 40% might not be important heterogeneity, an I2 between 30% to 60% may represent moderate heterogeneity and an I2 between 50% to 90% were considered to represent substantial heterogeneity. Finally, I2 =75–100% would involve considerable heterogeneity.31 We calculated the effect sizes with the standardised mean difference (SMD) for the strength variable, active ROM, TUG, and pain intensity given that they are expressed in different scales and units, and set the confidence intervals (CI) at 95%. The estimated SMDs were interpreted as described by Hopkins et al.32 (i.e., an SMD of 4.0 was considered to represent an extremely large clinical effect; 2.0–4.0 a very large effect; 1.2–2.0 a large effect; 0.6–1.2 a moderate effect; 0.2–0.6 a small effect; and 0.0–0.2 a trivial effect). A sensitivity analysis was performed to assess the possible influence of outliers and robustness of the results, where each study is removed one by one. In addition, a subgroup analysis was performed to assess the specific effect of the rehabilitation phase where MI is included. Phases were divided as follows: a) immediate term rehabilitation (up to 4 weeks after surgery); b) post-acute rehabilitation (from 3 to 12 months post-surgery). Criteria were based on expert consensus on best practices for rehabilitation after TKA.1 SMD was re-expressed to Mean Difference (MD) to facilitate clinical interpretation. For this purpose, the most representative study with the lowest risk of bias was selected for each variable and the standard deviation of the control group (end of study mean or mean change from baseline to end of study) by the pooled SMD was multiplied to obtain the re-expressed MD. Minimal detectable change (MDC) was used in the clinical interpretation of the results.
ResultsFig. 1 shows the study flow chart. Seven studies met the inclusion criteria and were included in this review. Table 1 presents the studies' characteristics with regards to sample size, population, intervention, outcomes, main results, and conclusions.
Study characteristics.
| Study | Participants | Intervention | Control | Variables | Conclusions |
|---|---|---|---|---|---|
| RCT pilot33 | 24 patients undergoing TKA from 65 to 75 years | N = 12Standard rehabilitation (3 times per week for 4 weeks) + MI exercises (15-min sessions) | N = 12Standard rehabilitation (3 times per week for 4 weeks) + Neutral activities (15-min sessions) |
| MI plus standard rehabilitation improves strength and functional recovery in patients with TKA |
| RCT pilot34 | 20 patients undergoing TKA from 65 to 75 years | N = 10Standard rehabilitation (3 times per week for 4 weeks) + MI exercises (15-min sessions) | N = 10Standard rehabilitation (3 times per week for 4 weeks) + Neutral activities (15-min sessions) |
| MI combined to standard rehabilitation improves quadriceps strength and knee flexion ROM in patients with TKA |
| RCT39 | 34 patients undergoing bilateral TKA from 50 to 85 years | N = 17Standard rehabilitation (4 weeks) + MI exercises (15-min sessions 5 days per week) | N = 17Standard rehabilitation (4 weeks) |
| MI plus standard rehabilitation improves strength and reduces its loss and gait speed in patients with TKA 4 weeks after surgery |
| RCT35 | 26 patients undergoing TKA from 50 to 85 years | N = 13Standard rehabilitation (5 times a week, 2 times per day for 4 weeks) + MI exercises (2 sets of 25 repetitions) | N = 13Standard rehabilitation (5 times a week, 2 times per day for 4 weeks) |
| The addition of MI to physical therapy rehabilitation preserved levels of strength and subjective measures of physical function |
| RCT36 | 48 patients undergoing TKA from 59 to 73 years | N = 24Standard rehabilitation + MI training (2 30-min sessions per day, every day along 11 days) | N = 24Standard rehabilitation + Training based on the enhancement of non-motoric cognitive functions (2 30-min sessions per day, every day along 11 days) |
| MI training combined to standard physical therapy rehabilitation may improve gait and limit new falls in the long term in patients with recent TKA in the post-surgery acute phase |
| RCT pilot37 | 10 patients undergoing unilateral TKA from 50 to 80 years | N = 4Standard rehabilitation (3 times per week for 2 months) + 10 mins of MI + Exercise programme | N = 6Standard rehabilitation (3 times per week for 2 months) + Exercise programme | - Knee flexion ROM- Pain intensity | MI plus standard rehabilitation after TKA may improve knee flexion ROM and function |
| RCT38 | 24 patients undergoing TKA from 60 to 85 years | N = 12Standard rehabilitation (5 30-min sessions + Exercise programme mentally performed) | N = 12Standard rehabilitation (5 30-min sessions + Exercise programme physically performed |
| MI combined to early physical therapy rehabilitation improves disability but no ROM in patients with TKA |
MI, motor imagery; RCT, randomised controlled trial, ROM, range of motion; TKA, total knee arthroplasty.
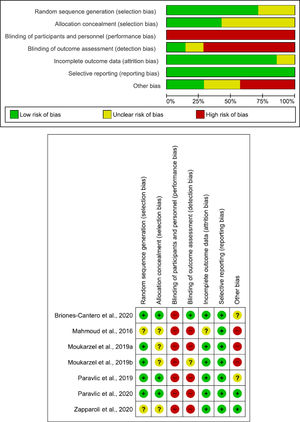
Most of the studies had a low risk of selective reporting bias. The domain with the highest percentage of studies with a high risk of bias was the blinding of participants and personnel (performance bias). Fig. 2 shows the summary of the risk of bias and the graph for the risk of bias. The inter-rater reliability of the Cochrane risk of bias tool was high (κ=0.733).
Study characteristicsRegarding the outcome measures, six studies assessed active ROM,33–38 four studies assessed quadriceps strength,33–35,39 four studies assessed TUG,33–36 and five studies assessed pain intensity.34–38 Five studies performed the intervention immediately after surgery,34–36,38,39 while two studies during the post-acute term.33,37 All included studies compared MI added to a standard physical therapy program against the same standard physical therapy program with33–36 or without37–39 an additional non-imagery activity. The standard physical therapy consisted of lower limb mobility and strengthening exercises, as well as physical agents and manual therapy.
Main results and certainty of evidenceQuadriceps strengthThe pooled effect showed a positive moderate effect in favour of MI (4 studies33–35,39; SMD: 0.88; 95% CI: 0.42, 1.34; I2=19%; re-expressed MD of quadriceps strength normalised to body mass35: 0.22 Nm/kg; MDC: Not available) (Fig. 3A). There is low certainty of evidence, downgraded due to imprecision and risk of bias, that MI may increase quadriceps strength in patients after TKA (Table 2).
Synthesis forest plot. Forest plot summarises the results of included studies (sample size, standardised mean differences [SMDs], and weight). The small boxes represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI). Favors [Intervention].
Summary of findings and quality of evidence (GRADE).
CI, confidence interval; MI, motor imagery; RCT, randomised controlled trial; ROM, range of motion; TUG, Time Up and Go.
The pooled effect showed a positive moderate effect in favour of MI (6 studies33–38; SMD: 0.62; 95% CI: 0.05, 1.19; I2=61%; re-expressed MD35: 8.85°; MDC40: 4.0°−5.8°) (Fig. 3B). This result is based on low certainty evidence, downgraded due to imprecision, risk of bias, and inconsistency. Hence, we are uncertain about whether MI increases ROM in patients after TKA (Table 2).
A sensitivity analysis showed that two studies significantly affected the pooled effect33,38 (p>0.05). A meta-analysis without these two articles showed no influence of these outliers in the pooled effect (SMD: 0.47; 95% CI: 0.05, 0.89; I2= 7%).
A sub-group analysis comparing studies that performed the intervention immediately after surgery versus the post-acute term was performed. For post-acute term intervention, pooled effect showed a positive large effect in favours of MI (2 studies33,37; SMD: 1.72; 95% CI: 0.9, 2.54; I2=0%; re-expressed MD35: 24.56°) (Fig. 3B). Subgroup analysis of studies that performed the intervention immediately after surgery showed no significant differences at immediate term (4 studies34–36,38; SMD: 0.27; 95% CI: −0.1, 0.63; I2=0%; re-expressed MD35: 3.86°) (Fig. 3B). There is low certainty evidence, downgraded due to imprecision and inconsistency, that MI may increase ROM when administered in the post-acute term but not when administered immediately after surgery.
Timed-up-go testThe pooled effect did not show any beneficial effect of adding MI to standard therapy (4 studies33–36; SMD: −0.59; 95% CI: −1.21, 0.03; I2=61%; re-expressed MD35: −1.78 s; MDC41: 2.27 s) (Fig. 3C). This result is based on very-low certainty evidence, downgraded due to imprecision, risk of bias, and inconsistency. So, we are uncertain about whether MI increases TUG in patients after TKA (Table 2).
Pain intensityThe summary effects showed a positive small effect for the MI intervention in five studies33,35–38 (SMD: −0.51; 95% CI: −0.92, −0.10; I2=38%; re-expressed MD35: −7.48 mm; minimal clinically significant difference42: 12 mm) (Fig. 3D). There is low certainty evidence, downgraded due to imprecision and risk of bias, that MI may reduce pain intensity slightly in patients after TKA (Table 2).
DiscussionThe main aim of the present systematic review and meta-analysis was to investigate the effects of MI on clinical outcomes in patients with TKA. Our results showed that there is low certainty evidence that adding a MI intervention to standard rehabilitation may increase knee strength and reduce pain. However, it is uncertain whether MI can improve active ROM and mobility. Furthermore, our findings suggest that when MI is performed in the post-acute term it may improve active ROM.
Quadriceps strength is one of the most relevant outcome measures in patients with osteoarthritis and after TKA. Loss of quadriceps strength after TKA surgery is a common occurrence, and studies have reported a central activation deficit that could contribute to long-term muscle weakness.43,44 In addition, active ROM is a critical functional variable to consider after TKA.37,45 Movement representation methods, specifically MI, could therefore play an important role in patients after TKA. MI might activate central movement patterns similar to actual exercise and could promote central activation and a motor learning process that could be related to motor impairments after surgery.46,47
Several neurophysiological theories have been developed regarding the effects of MI on variables such as those included in our review. It has been proposed that MI promotes an adaptive neuroplastic process that could influence motor recruitment and synchronisation of motor units at the peripheral level.34 Movement mental construction could improve motor force generation representation at the central level, improving cortical planning and, subsequently, peripheral motor performance.48,49 Other neurophysiological aspects, such as modulation of corticospinal excitability and autonomic nervous system participation, could be related to motor changes following MI.50 The benefits of MI on motor performance have been previously described.51 The results obtained in this research are consistent with those of Nicholson et al.15 or Cuenca-Martínez et al.51 regarding the benefits of MI on motor variables in clinical population. In recent years, there has been growing interest in this technique in rehabilitation sciences.52 Motor imagery has been shown to be an alternative for improving motor variables in various populations, such as patients with musculoskeletal pain,53 those with stroke54 and those undergoing neurological rehabilitation.55 In the same way that improvements have been observed in variables related to motor function, improvements in pain intensity levels have been seen with MI interventions. Pain relief has already been reported in some MI studies of patients with chronic pain with other anatomical region involvement.56,57
However, we did not find significant improvements from MI on functional capacity variables such as the TUG. These types of variables involve patient's postural stability, gait, stride length, and sway.58 This is probably due to the multitude of simultaneous processes involving different processing areas, which have not been specifically trained with MI interventions. In populations with neurological pathology, by training with MI specifically using tasks specifically related to the activities such as the TUG, improvements in this variable have been observed.59
Paravlic et al.60 had published a review in 2020 with a meta-analysis on the general effects of movement representation methods on leg arthroplasty. Consistent with our results, the authors found that various interventions, such as MI, action observation, guided imagery, and implicit imagery added to standard rehabilitation could be effective in improving functionality. In contrast to this previous review, we focused our study on motor variables such as quadriceps strength and active ROM. We also included new studies that precisely determine the effect of MI on patients after TKA.
One of the most characteristic aspects of an MI intervention, in contrast to other representation methods, is that it requires individual internal construction of the movement. The ability to imagine can determine the therapeutic efficacy of this intervention, given that it appears to be highly dependant on the ability to construct the image.61 Despite this, MI could be the most effective strategy in these patients, although we need to determine the role of imaging ability and consider it from a clinical point of view to optimise results.
LimitationsThis study has a number of limitations to be considered. First, although we followed a systematic search strategy, the risk of selection bias might still be present. Second, the low number of studies included in the review and meta-analysis could represent inadequate statistical power and bias due to the small sample size included in each comparison. In addition, most of the studies did not include a placebo intervention in addition to the standard treatment, which makes it difficult to determine whether the effects were driven by movement representation techniques and not due to nonspecific effects. Third, there is great variability in the interventions and measurement procedures used amongst the studies. Also, one of the included studies was a pilot trial published as a thesis, which could lead to a bias when interpreting its results.37 We could not confidently assess the risk of publication bias because of the small number of included studies, which could have biased our results. Another limitation to take into account is that after registration in the PROSPERO platform prior to the study, the manuscript underwent some modifications related to the analysis of the results due to recommendations made during the editorial process to improve the presentation of the results obtained in the review. Lastly, methodological concerns regarding the studies included, specially referred to performance and detection bias should be considered when interpreting results.
ConclusionsThe present review and meta-analysis showed the positive effects of MI in restoring quadriceps strength, active ROM, and decrease pain intensity in patients after TKA. There is low evidence that adding MI to a standard rehabilitation programme for patients with TKA likely improve quadriceps strength and decrease pain intensity but not physical function. According to the quality of evidence, it is uncertain whether MI improve active ROM. This study's findings should be considered with caution due to the low number of studies, and future studies are needed to obtain stronger conclusions.
The authors would like to thank the CSEU La Salle for its services in editing this manuscript.
Prospero registration: CRD42020205353.







![Synthesis forest plot. Forest plot summarises the results of included studies (sample size, standardised mean differences [SMDs], and weight). The small boxes represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI). Favors [Intervention].](https://static.elsevier.es/multimedia/14133555/0000002500000006/v1_202112250704/S1413355521001040/v1_202112250704/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90NRiOl/KnYC22JyCfllZoM=)





