Different exercises have gained much interest for managing post-COVID-19 pulmonary fibrosis (PC19-PF).
ObjectiveTo compare the impacts of aerobic, resistance, and combined exercises on ventilatory function, lung fibrosis, exercise capacity, and quality of life (QoL) in men with chronic PC19-PF.
MethodsEighty males with chronic PC19-PF aged 40–60 were randomly assigned to four groups: aerobic exercise (AE), resistance exercise (RE), AE/RE, and a control group. Outcomes included forced vital capacity (FVC), diffusing capacity of the lungs for carbon monoxide (DLCO), fibrosis grade by computed tomography, exercise capacity using the estimated maximal oxygen consumption (VO2max), dyspnea using the Medical Research Council (MRC) dyspnea scale, and QoL using the 12-item Short Form Health Survey (SF-12).
ResultsFor all outcome measures, significant group by time interactions were noted (p < 0.05). The AE and AE/RE groups demonstrated significant improvements in all outcomes compared to controls (p < 0.05), with no notable differences between the two groups, except for the estimated VO2max, in favor of AE (p = 0.04). Compared to controls, RE significantly improved the estimated VO2max, dyspnea (MRC scale), and QoL (p < 0.05) with no effects on FVC, DLCO, or fibrosis grade (p > 0.05). In addition, compared to RE, both AE and AE/RE significantly improved all outcomes (p < 0.05).
ConclusionIn men with chronic PC19-PF, both AE and AE/RE could similarly improve ventilatory function, lung fibrosis, dyspnea, and QoL, with AE improving exercise capacity most.
Post-COVID-19 pulmonary fibrosis (PC19-PF) refers to persistent fibrotic changes in the lungs with associated functional limitations following COVID-19 infection.1 It is a major cause of long-term respiratory morbidity,2 affecting approximately 44.9 % of COVID-19 survivors,3 with a prevalence 1.3 times higher in males than in females.4 PC19-PF may result from pneumonia and acute respiratory distress syndrome during the acute phase of COVID-19,5 with increased severity in aged individuals, patients with chronic comorbidities, and those requiring mechanical ventilation.6,7 Symptoms of PC19-PF include difficulty breathing, dry cough, and low oxygen saturation, which can significantly affect health and quality of life (QoL).1 Lung function tests frequently show persistent impairments in diffusion capacity for carbon monoxide (DLCO) and a restrictive pattern,8 commonly associated with radiological fibrotic alternations.9
Pulmonary rehabilitation has demonstrated efficacy in alleviating symptoms and improving QoL in individuals with PC19-PF.10 In such cases, aerobic exercise (AE) added to diaphragmatic breathing exercise (DBE) enhances ventilatory function and exercise capacity, along with reducing dyspnea.11 Furthermore, combined aerobic and resistance training enhances pulmonary function, functional capacity, and QoL while reducing dyspnea in those with PC19-PF.12 Resistance exercise (RE) has also been found to improve ventilatory function and exercise performance in patients post-COVID-19.13 However, none of these studies utilized randomized controlled trial designs.11–13
Due to the lack of trials assessing the health benefits of different exercise modalities for patients with PC19-PF, elucidating the comparative advantages of these interventions can guide rehabilitation protocols. This interventional research aimed to compare the relative benefits of AE, RE, and a combination of both on ventilatory function (the primary focus), as well as fibrosis grading, exercise capacity, dyspnea, and QoL (secondary aspects) among men with PC19-PF.
MethodsStudy settings and ethical considerationsThis trial, conducted from March to June 2024, followed the CONSORT 2010 guidelines14 and utilized a parallel-group, single-center, prospective, randomized-controlled design. No changes were made to the study protocol after trial commencement. Participants were recruited from Al Mahalla Al Kobra Chest Hospital in Egypt through referrals from chest physicians. Interventions were performed in the physical therapy unit of the hospital. The study protocol was approved by the Ethics Committee Centre for Human Scientific Research at Cairo University in Egypt (approval number P.T.REC/012/005166). The study procedures adhered to the Helsinki Declaration principles, and informed consents were obtained from all participants.
Sample size estimationThe sample size was determined using the G Power 3.1 software based on the forced vital capacity (FVC) outcome measure of Essam et al.'s study.15 A one-way ANOVA test with a chance of error (α) of 5 %, a power of 85 %, and an effect size of 0.45, as derived from the study of Essam et al., indicated a sample of 19 participants per group. To account for attrition, 20 participants were assigned per group.
Randomization, allocation, and blindingSimple randomization was performed using a computer-generated sequence, with participants allocated equally (1:1:1:1) to the control, combined training, AE, and RE groups. Recruitment was conducted by two blinded physical therapists using sequentially numbered opaque envelopes to mitigate allocation bias. Blinding of participants and exercise supervisors was not possible because of the characteristics of the interventions. However, the chest physician prescribing medications and ventilatory function and the fibrosis assessors and statistician were blinded to group allocation. In contrast, assessors of exercise capacity, dyspnea, and QoL were unblinded due to practical considerations.
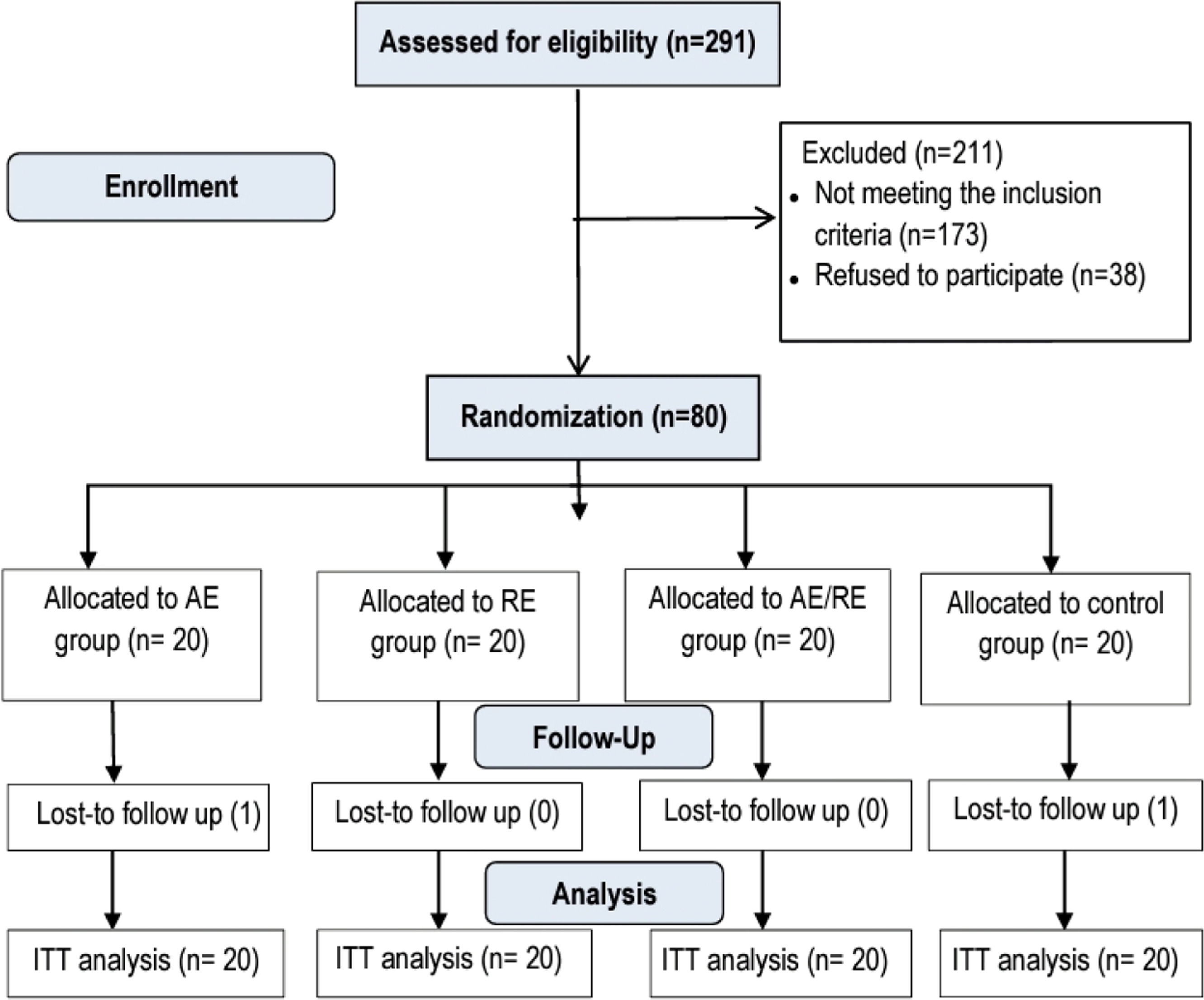
ParticipantsThe study included 80 males. Inclusion criteria comprised mild-to-moderate PC19-PF, scoring 1–3 on the PC19-PF grading system;16 a history of moderate-to-severe COVID-19 infection, scoring 4–9 on the World Health Organization clinical progression scale17; persistent fibrosis lasting over 6 months; mild and moderate dyspnea, scoring 1–3 on the dyspnea scale of the Medical Research Council (MRC)18; age 40–60 years and body mass index 18.5–29.9 kg/m². Exclusion criteria included any other chronic chest disease, unstable cardiovascular disorders, smoking, oxygen support, neurological or musculoskeletal disorders affecting exercise performance, and other health conditions inappropriate for the study, as confirmed by chest physicians. Participants were randomly assigned to four equal groups: AE (AE and DBE), RE (RE and DBE), AE/RE (AE/RE and DBE), and control (DBE only). Fig. 1 shows development throughout the course of the trial.
EvaluationsAll outcome measures were assessed twice: at baseline and after 12 weeks.
Ventilatory function (Primary outcome)Ventilatory function parameters of FVC and DLCO were measured using the Spirolab III spirometer (MIR, Italy) following the American Thoracic Society guidelines.19 Following a 15-minute rest period, participants received a briefing on the procedure. For FVC, the mouthpiece and nose clip were fitted, and participants performed maximal inhalation followed by forced expiration. After resting for 10 min, DLCO was measured via deep inhalation, breath-holding for 10 s, and complete exhalation followed by tidal breath. The highest values from three trials for both FVC and DLCO were recorded as percentages of predicted normal values. Normal DLCO = 75 %−140 % while normal FVC = 80 %−100 %.20
Secondary outcomesFibrosis gradingFibrosis grading was conducted by an experienced radiologist using a high-resolution chest computed tomography (CT) scanner (Ingenuity Core 128, Philips, Netherlands) without contrast. Axial plane scans were conducted from the lung apex till the diaphragm at full inspiration. Lung fibrosis was assessed according to the Demircioglu et al. method, categorizing PC19-PF into five grades: 0 (normal), 1 (mild fibrosis), 2 (reticulation), 3 (linear streaks or parenchymal bands), and 4 (deformation with volume loss).16
Exercise capacityThe modified Bruce protocol was used for maximal symptom-limited treadmill exercise testing.21 This protocol is more appropriate for individuals with reduced exercise capacity than the standard Bruce protocol.22 Participants performed the test on a Phantom AC6069m treadmill (Taiwan) until maximal exertion. Heart rates were continuously monitored with a Granzia fingertip oximeter (Italy), and indicators for test termination were checked. The recorded maximal heart rate (Hrmax) was utilized to determine AE intensity. For exercise capacity assessment, duration to exhaustion was recorded to estimate maximal oxygen consumption (VO2max) using the formula: estimated VO2max = 2.94 × (time to exertion in minutes) + 7.65.23
DyspneaDyspnea severity was assessed using the simple and validated MRC dyspnea scale24 through face-to-face interviews. The scale possesses five grades: Grade 1 (breathless only after severe effort), Grade 2 (dyspnea when rushing on a flat surface), Grade 3 (needing to stop for breathing), Grade 4 (difficulty breathing after 100 yards), and Grade 5 (too breathless to go outside).18
Quality of life (QoL)This study utilized the Arabic version of the 12-Item Short Form Health Survey (SF-12) to assess QoL,25 verified and translated from the original version.26 Patients were asked to complete the SF-12 during in-person interviews. The survey consists of 12 questions, yielding two scores: the physical component score (PCS) and the mental component score (MCS). According to Ware et al., a greater PCS score indicates improved physical health, while a greater MCS score denotes enhanced mental health.27
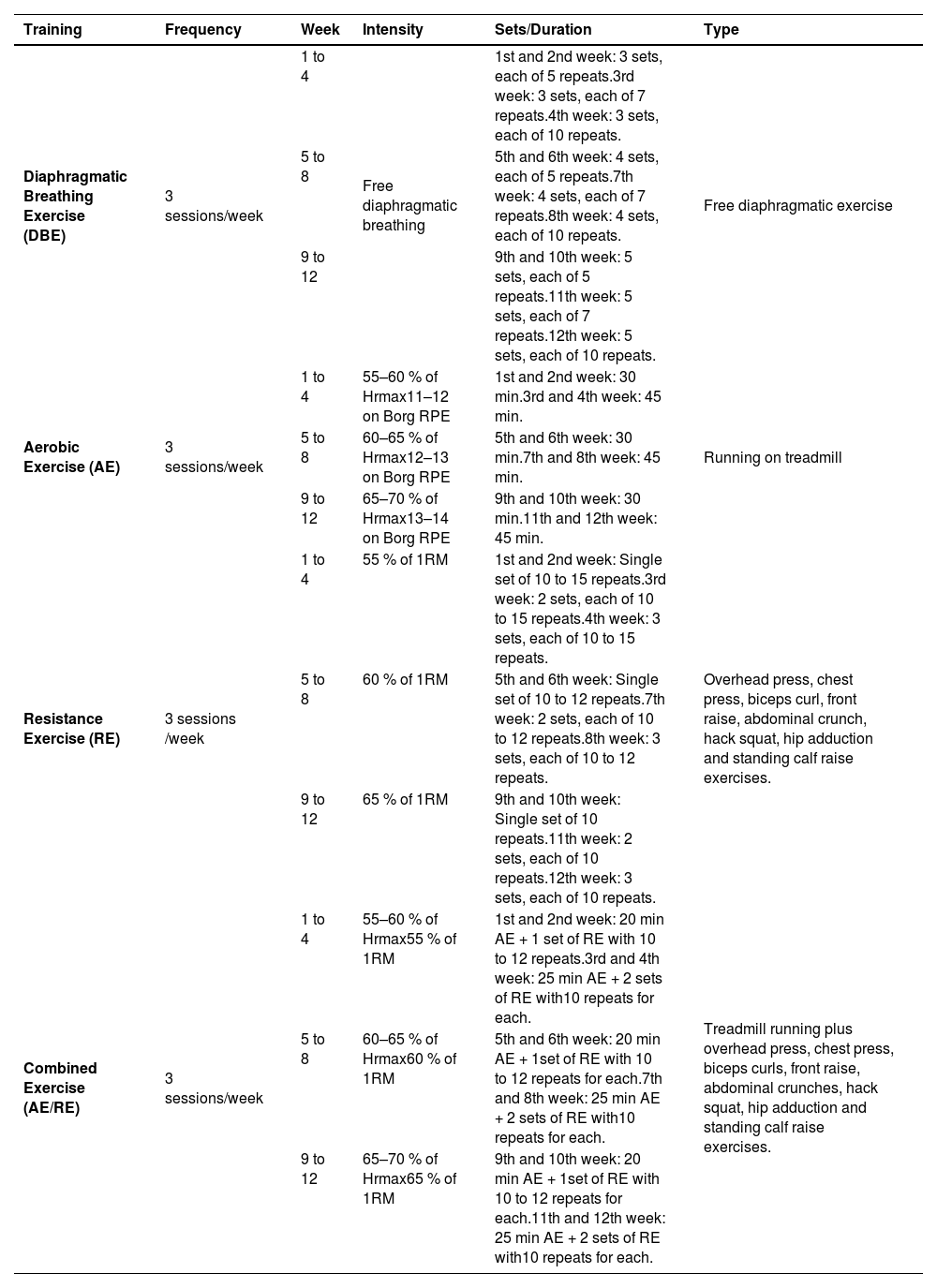
Exercise interventionsAn experienced physical therapist designed and supervised the training protocols, following the FITT principle established by the American College of Sports Medicine.28 All exercises were scheduled three times weekly for 12 weeks, as outlined in Table 1.
Exercise protocols.
Abbreviations: Hrmax, maximal heart rate; Borg RPE, Borg rating of perceived exertion 20-point scale; 1RM, one repetition maximum.
All participants performed DBE starting in the hook-lying position, with hands placed on the abdomen below the costal cartilage. Patients were instructed to breathe deeply, expanding the abdomen while keeping the chest and shoulders relaxed. Each cycle comprised a 3-second inhalation through the nose, a 3-second pause, and a 6-second exhalation through pursed lips.29 The protocol included 3–5 sets of 5–10 deep breaths with 2-minute rest periods between sets.
Aerobic exercise (AE)Participants in the AE group completed 30–45 min of treadmill running at low-to-moderate intensity. Intensity was sustained by maintaining the heart rate within 55 % and 70 % of Hrmax and perceived exertion between 11 and 14 on the Borg 20-point scale.30 A Pulsox-304 pulse oximeter monitored oxygen saturation and heart rate for adherence to the training zone. Each session involved a 10-minute warm-up, a 3-minute cool-down, with the program progressively performed by increasing duration and, subsequently, intensity.28,31
Resistance exercise (RE)The RE group participated in resistance forms of overhead presses, chest presses, biceps curls, front raises, abdominal crunches, hack squats, hip adductions, and standing calf raises, using free weights and gym machines at low-to-moderate intensity of 55–65 % of each participant’s one-repetition maximum (1RM), assessed as the maximum successfully lifted weight in a single lifting trial.32 Sessions consisted of a 10-minute warm-up stretching, 20–50 min of exercise, and a 5-minute cool-down stretching. Participants did 1–3 sets of 10–15 repeats for each exercise with 10 s of passive rest between repetitions and 2 min between sets. Progression was done with increasing sets before adding resistance.
Combined aerobic and resistance exercise (AE/RE)The combined training group performed mild-to-moderate aerobic (55–70 % Hrmax) and resistance (55–65 % 1RM) workouts as outlined in the single training form. Each session commenced with stretching for 10 min, followed by 15–25 min of AE, then 10–25 min of RE. A 5-minute cool-down through stretching concluded each session.
Analysis of dataData were analyzed using SPSS software (version 22, Inc., Chicago). Variance homogeneity and data normality were confirmed through Levene's and Shapiro-Wilk tests, respectively (p > 0.05). One-way ANOVA was employed to compare demographic and anthropometric characteristics across the four groups. A mixed 4 × 2 factorial ANOVA assessed group (AE, RE, AE/RE, control) × time (pre- and post-intervention) interactions for all outcomes. Post-study pairwise comparisons used the Bonferroni test. Between-group changes were evaluated with 95 % confidence intervals (CI), mean differences (MD), and Cohen's effect size (d), which was assessed using the standardized difference among two means33 and categorized as minor (d = 0.2), moderate (d = 0.5), or large (d = 0.8).34 The Kruskal-Wallis test was applied for categorical variables. All tests used a significance level of 0.05. Missing COVID duration data for certain patients were imputed using the expectation-maximization method,35 and the intention-to-treat approach was implemented.
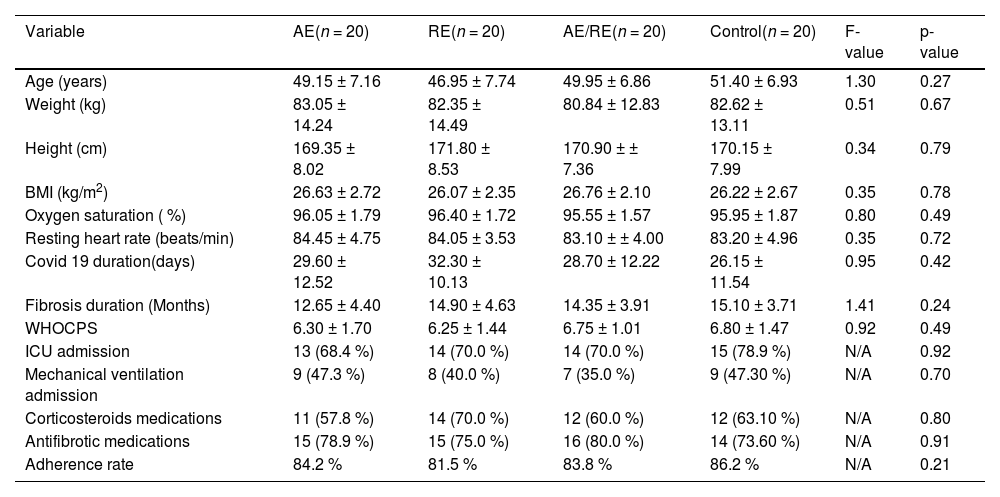
ResultsOf the 80 participants, one withdrew from the AE group at their request, and one dropped out in the control group due to kidney-related issues. No significant differences were observed among groups at baseline regarding demographic, anthropometric, and clinical traits, as well as session adherence (p > 0.05) (Table 2).
Baseline characteristics of participants (all males).
Data are shown as means ± standard deviations for continuous measures and absolute frequency ( %) for categorical measures.
Abbreviations: AE, aerobic exercise; RE, resistance exercise; BMI, body mass index; WHOCPS, world health organization clinical progression scale; ICU, intensive care unit; N/A, not applicable.
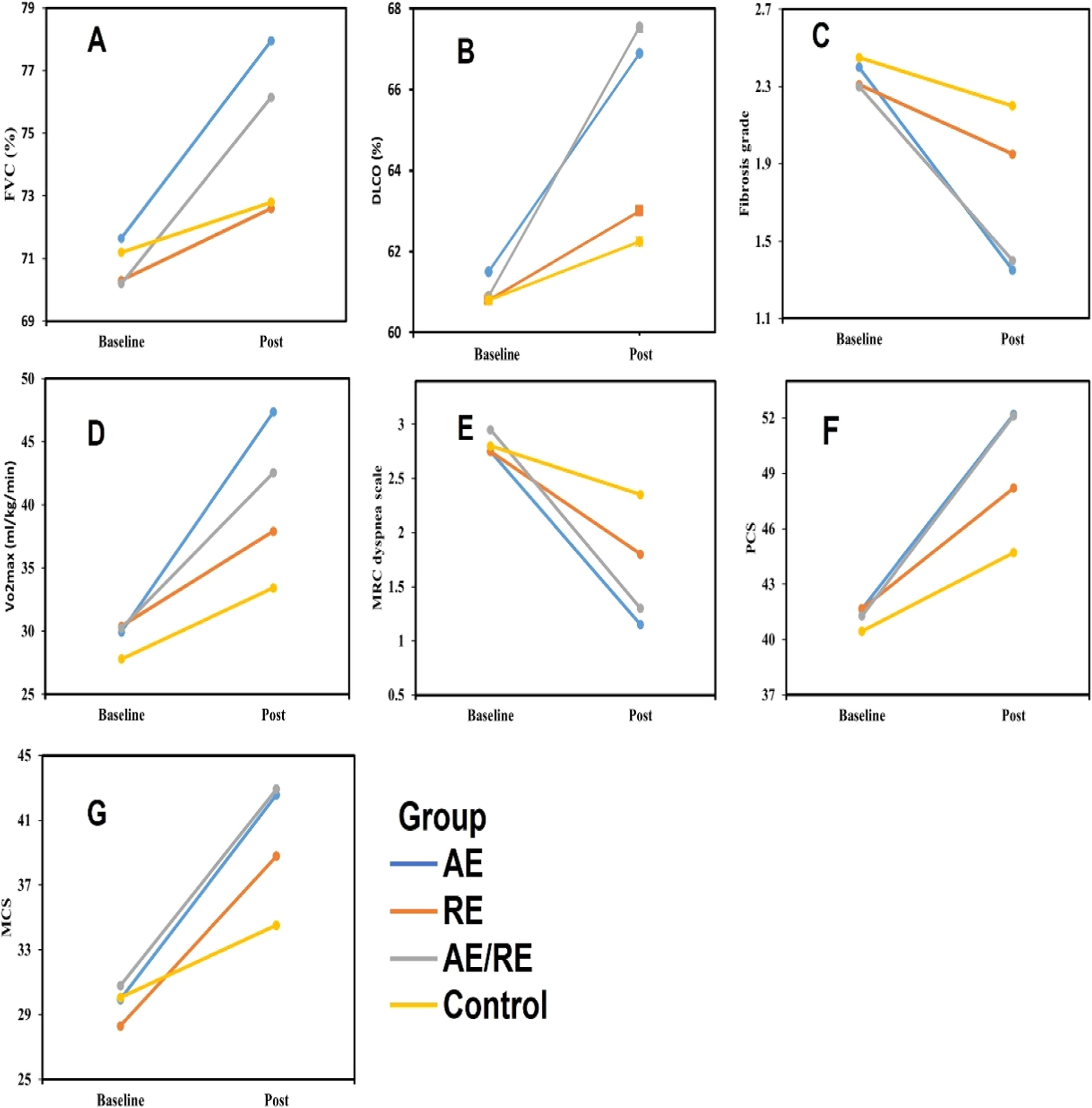
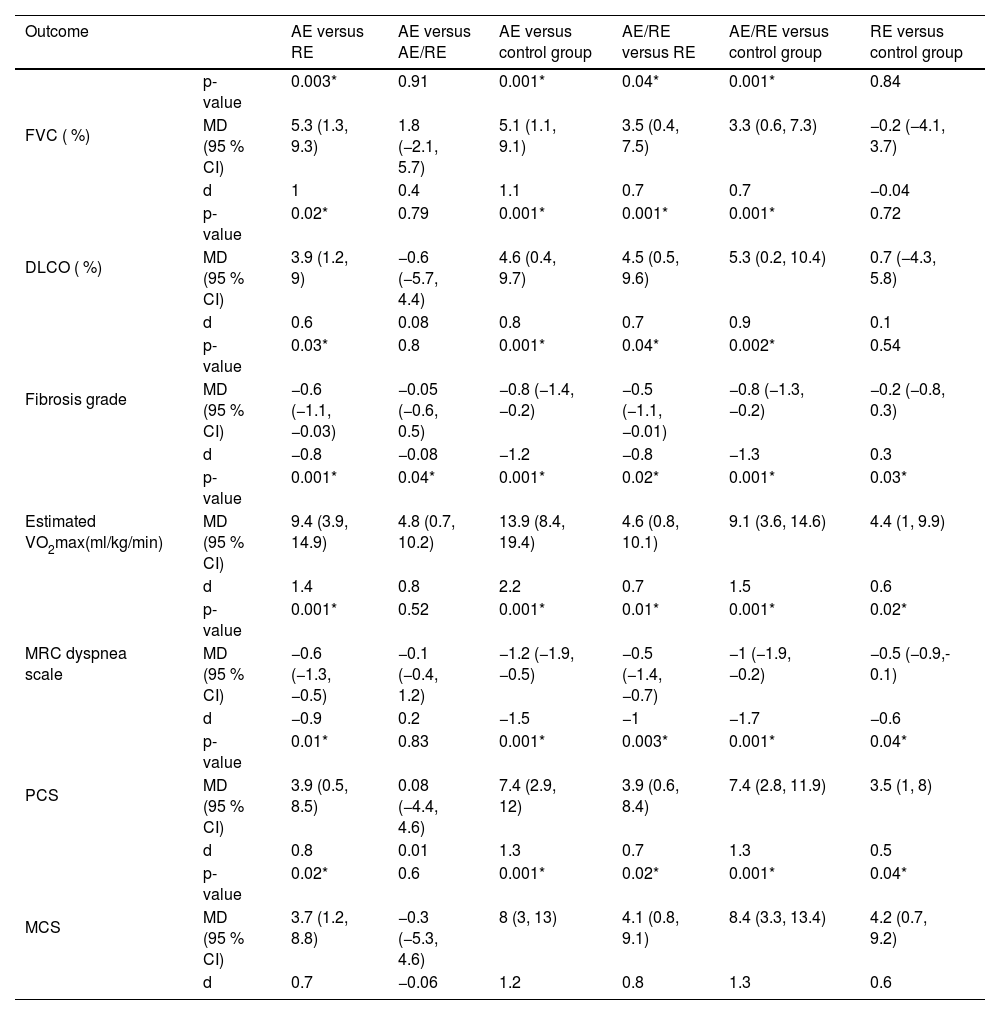
The mixed ANOVA showed a significant group × time interaction for both FVC (p = 0.001) and DLCO (p = 0.001). All groups exhibited significant enhancements in both measures (p < 0.05), as demonstrated in Figs. 2A and 2B. AE led to greater FVC rises compared to RE (MD = 5.3 %; 95 % CI = 1.3, 9.3; p = 0.003) and the control group (MD = 5.1 %; 95 % CI = 1.1, 9.1; p = 0.001) (Table 3). Furthermore, AE/RE exhibited more significant FVC increases than RE (MD = 3.5 %; 95 % CI = 0.4, 7.5; p = 0.04) and control (MD = 3.3 %; 95 % CI = 0.6, 7.3; p = 0.001) (Table 3). AE also improved DLCO more than RE (MD = 3.9 %; 95 % CI = 1.2, 9; p = 0.02) and control (MD = 4.6 %; 95 % CI = 0.4, 9.7; p = 0.001) (Table 3). AE/RE demonstrated greater DLCO improvements than RE (MD = 4.5 %; 95 % CI = 0.5, 9.6; p = 0.001) and control (MD = 5.3 %; 95 % CI = 0.2, 10.4; p = 0.001) (Table 3). For both measures, no significant variations were noted between AE and AE/RE or between RE and control (p > 0.05) (Table 3).
Baseline and post-intervention FVC (A), DLCO (B), Fibrosis grade (C), Vo2max (D), MRC dyspnea scale (E), PCS (F), and MCS (G) .
Values are means.
Post-study pairwise comparisons between groups.
Data are shown as p-values, Mean difference (MD), Cohen’s effect size (d), and 95 % confidence interval (CI).
* Significant variation among groups (p-value < 0.05).
Abbreviations: AE, aerobic exercise; RE, resistance exercise; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide; VO2max, maximal oxygen consumption; SF-12, 12-Item Short Form Health Survey; PCS, physical component score; MCS, mental component score.
A significant group × time interaction was noted for fibrosis grades (p = 0.003). Significant reductions were noted in the AE and AE/RE groups (p < 0.05), whereas the RE and control groups exhibited no substantial changes (p > 0.05), as depicted in Fig. 2C. AE showed greater reductions in fibrosis compared to RE (MD = −0.6; 95 % CI = −1.1, −0.03; p = 0.03) and the control group (MD = −0.8; 95 % CI = −1.4, −0.2; p = 0.001) (Table 3). Similarly, AE/RE showed more significant fibrosis reduction compared to RE (MD = −0.5; 95 % CI = −1.1, −0.01; p = 0.04) and the control group (MD = −0.8; 95 % CI = −1.3, −0.2; p = 0.002) (Table 3). No significant variations were noted between the AE and AE/RE groups, nor between the resistance and control groups, regarding post-intervention fibrosis grades (p > 0.05) (Table 3).
Exercise capacityThe results showed a significant group × time interaction for the estimated VO2max (p = 0.001), with significant increases in all groups (p < 0.05), as shown in Fig. 2D. AE demonstrated greater increase in the estimated VO2max compared to RE (MD = 9.4 ml/kg/min; 95 % CI = 3.9, 14.9; p = 0.001) and the control group (MD = 13.9 ml/kg/min; 95 % CI = 8.4, 19.4; p = 0.001) (Table 3). AE/RE also showed more significant increases than RE (MD = 4.6 ml/kg/min; 95 % CI = 0.8, 10.1; p = 0.02) and the control group (MD = 9.1 ml/kg/min; 95 % CI = 3.6, 14.6; p = 0.001) (Table 3). AE showed significantly more improvement than AE/RE (MD = 4.8 ml/kg/min; 95 % CI = 0.7, 10.2; p = 0.04) (Table 3). RE also showed more significant increases than the control group (MD = 4.4 ml/kg/min; 95 % CI = 1, 9.9; p = 0.03) (Table 3).
DyspneaThe mixed ANOVA revealed a significant group × time interaction for the MRC dyspnea scale (p = 0.003), with significant declines in all groups (p < 0.05), as shown in Fig. 2E. AE exhibited more significant reductions in the MRC score compared to RE (MD = −0.6; 95 % CI = −1.3, −0.5; p = 0.001) and the control group (MD = −1.2; 95 % CI = −1.9, −0.5; p = 0.001) (Table 3). AE/RE also showed more significant declines compared to RE (MD = −0.5; 95 % CI = −1.4, −0.7; p = 0.01) and the control group (MD = −1; 95 % CI = −1.9, −0.2; p = 0.001) (Table 3). No notable variation was recorded between AE and AE/RE (p > 0.05) (Table 3). RE showed more significant declines than the control group (MD = −0.5; 95 % CI = −0.9, −0.1; p = 0.02) (Table 3).
Quality of lifeA significant group × time interaction was recorded for both PCS (p = 0.04) and MCS (p = 0.005), with significant increase in all groups for both scores (p < 0.05), as shown in Figs. 2F and 2G. AE showed more significant increases in PCS compared to RE (MD = 3.9; 95 % CI = 0.5, 8.5; p = 0.01) and the control group (MD = 7.4; 95 % CI = 2.9, 12; p = 0.001), as well as in MCS compared to RE (MD = 3.7; 95 % CI = 1.2, 8.8; p = 0.02) and the control group (MD = 8; 95 % CI = 3, 13; p = 0.001) (Table 3). Similarly, AE/RE showed greater improvements in both PCS and MCS compared to RE (PCS: MD = 3.9; 95 % CI = 0.6, 8.4; p = 0.003; MCS: MD = 4.1; 95 % CI = 0.8, 9.1; p = 0.02) and the control group (PCS: MD = 7.4; 95 % CI = 2.8, 11.9; p = 0.001; MCS: MD = 8.4; 95 % CI = 3.3, 13.4; p = 0.001), with no significant differences between AE and AE/RE (p > 0.05) (Table 3). Also, RE demonstrated notable increases in PCS and MCS compared to the control group (PCS: MD = 3.5; 95 % CI = 1, 8; p = 0.04; MCS: MD = 4.2; 95 % CI = 0.7, 9.2; p = 0.04) (Table 3).
Adverse eventsThe interventions did not result in any adverse effects.
DiscussionThis study reports a comparison of the effects of AE, RE, and their combination on men with persistent PC19-PF. Key findings include: (i) AE and AE/RE significantly improved ventilatory function, fibrosis, exercise capacity, dyspnea, and QoL compared to the control group; (ii) AE and AE/RE showed similar improvements, except for exercise capacity; (iii) RE improved exercise capacity, dyspnea, and QoL but did not affect ventilatory function or fibrosis; (iv) AE and AE/RE outperformed RE in all outcomes; (v) AE yielded the greatest improvement in exercise capacity, followed by AE/RE, with RE showing the least improvement.
AE enhances oxidative enzyme function by increasing reactive oxygen and antioxidant proteins in respiratory tissues, inhibiting isoprostane levels,36 and boosting oxidative enzyme activity.37 It also reduces angiotensin-converting enzyme 2 expression in the lungs,38 potentially alleviating fibrosis.39 Additionally, AE increases respiratory demand and muscle stretching, improving respiratory muscle efficiency and lung compliance.40 DBE likely contributed to the improvements by enhancing ventilation, increasing tidal volume, and reducing dead space,41,42 which may explain the control group’s improvements.
The greater FVC and DLCO improvements with AE (d = 1 and 0.6, respectively) and AE/RE (d = 0.7) over RE exceeded the minimal clinically important difference (MCID) for FVC (i.e., 3 %)43, but not for DLCO (i.e., 11 %).44 This may be due to AE’s ability to recruit more alveoli, enhance oxygen tension, and reduce ventilation/perfusion mismatch, thereby boosting VO2max and reducing dyspnea.45,46 AE improves cardiopulmonary fitness, whereas RE focuses on muscular strength.47 Similar improvements in FVC, DLCO, MRC dyspnea scale, and functional capacity were reported in PC19-PF patients undergoing pulmonary rehabilitation with AE/RE and DBE at home12 and in hospitals.48 Notably, wide 95 % CIs for FVC and DLCO in comparisons of AE or AE/RE with RE suggest variability in pulmonary adaptations to these interventions, highlighting the need for an individualized intervention approach. In context, adding RE to DBE did not significantly improve FVC (d = −0.04, p = 0.84) or DLCO (d = 0.1, p = 0.72) compared to DBE alone. Similarly, eight weeks of moderate RE did not increase FVC in healthy women49 or sedentary men.50 AE and AE/RE also led to greater reductions in the MRC dyspnea scale than RE (d = −0.9 and −1, respectively), with relatively narrow 95 % CIs, indicating consistent improvements across groups.
Unsurprisingly, AE resulted in greater estimated VO2max enhancements than AE/RE (d = 0.8, p = 0.04), with a MD of 4.8 ml/min/kg, exceeding the MCID of 3.5 ml/min/kg.51 AE/RE also showed greater estimated VO2max improvements than RE (d = 0.7, p = 0.02), exceeding the MCID.51 Although the 95 % CIs for these comparisons were moderately wide, the consistent effect sizes reinforce the benefits of aerobic training. Similar findings were observed in obese individuals, where AE improved VO2max more than RE.52 A 22-week AE program also led to greater VO2max gains than AE/RE or RE in post-pubertal obese adolescents.53 The benefits of RE on VO2max, compared to controls, may stem from its ability to reduce muscle dysfunction in chronic respiratory diseases, improving exercise capacity, dyspnea, and QoL after −854,55 and 12-week programs.56
Long-term lung injury, particularly PC19-PF, negatively impacts QoL,57,58 correlating with fibrosis severity and lung function.59 This supports the superiority of AE and AE/RE over RE, as their mean between-group differences exceeded the MCID for SF-12 PCS and MCS components.60 However, the moderately wide 95 % CIs for these comparisons highlight variability in the observed responses. Such variability may limit the generalizability of the results to broader populations. A review of seven studies similarly reported that moderate AE/RE improved functional capacity and QoL in post-COVID-19 patients.61 Additionally, older adults with obstructive lung disorders demonstrated significant QoL improvements after adding AE to DBE over six months.62
This research provides a novel contribution to exercise therapy for PC19-PF. Limitations include the inability to directly measure VO2max and using a pulse oximeter instead of a heart rate monitor due to equipment constraints. Unblinded assessors for exercise capacity, dyspnea, and QoL may have affected results. The lack of female participants limits generalizability, and QoL was assessed with the general SF-12 due to the lack of an Arabic disease-specific tool. Additionally, uncontrolled factors like emotional variables or life challenges may have impacted QoL assessments.
ConclusionIn men with chronic post-COVID lung fibrosis, both AE and AE/RE, at low-to-moderate intensity, could lead to similar, greater improvements in ventilatory function, fibrosis grade, dyspnea, and QoL compared to RE. The AE may boost exercise capacity the most in such people, followed by the AE/RE, and then the RE. These findings indicate that exercise training may serve as a beneficial adjunctive approach for the management of chronic post-COVID lung fibrosis in men. However, further randomized trials with larger, more diverse cohorts are needed to validate and enhance these results.
Registration of the studyThe study has been prospectively registered with the Pan African Clinical Trial Registry (PACTR) with a registration number of PACTR202401627549473, available at https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=27170.
FundingThis study did not receive funding from any specific grant or organization.
The authors declare no competing interest.
The authors extend their appreciation to chest physicians and physical therapists at the intervention and recruitment sites.