Physical exercise has been used to mitigate the metabolic effects of diabetes mellitus.
ObjectiveTo evaluate the effect of resistance exercise when compared to aerobic exercise without insulin therapy on metabolic and clinical outcomes in patients with type 2 diabetes mellitus.
MethodsPapers were searched on the databases MEDLINE/PubMed, CINAHL, SPORTDiscus, LILACS, and SCIELO, without language or date of publication limits. Clinical trials that compared resistance exercise to aerobic exercise in adults with type 2 diabetes mellitus who did not use insulin therapy were included. The quality of evidence and risk of bias were assessed using the GRADE system and the Cochrane Risk of Bias tool, respectively. Meta-analysis was also used, whenever possible. Two reviewers extracted the data independently. Eight eligible articles were included in this study, with a total of 336 individuals, with a mean age of 48–58 years. The protocols of aerobic and resistance exercise varied in duration from eight to 22 weeks, 30–60min/day, three to five times/week.
ResultsOverall the available evidence came from a very low quality of evidence and there was an increase in Maximal oxygen consumption (mean difference: −2.86; 95% CI: −3.90 to −1.81; random effect) for the resistance exercise and no difference was found in Glycated hemoglobin, Body mass index, High-density lipoprotein cholesterol, Low-density lipoprotein cholesterol, triglycerides, and total cholesterol.
ConclusionsResistance exercise appears to be more effective in promoting an increase in Maximal oxygen consumption in protocols longer than 12 weeks and there is no difference in the control of glycemic and lipid levels between the two types of exercise.
Increasing life expectancy associated with a change in lifestyle contributed to the prevalence of chronic degenerative diseases, especially diabetes mellitus (DM). Data from the International Diabetes Federation1 estimate that more than 387 million people worldwide are diagnosed with this disease and by 2035 this number will rise to 592 million people. About 90% of the population with DM is affected by type 2 form.1
The relationship between regular physical activity, a proper diet, and restriction in the use of tobacco and alcohol is being increasingly discussed and scientifically analyzed to improve the quality of life of this population. There is already a consensus that physical activity has positive effects on prevention and/or maintenance of glycemic control and on the cardiovascular risk factors in this type of patient.2,3
In this sense, there are systematic reviews that compare resistance exercise versus control and aerobic exercise versus control, highlighting controversies about the benefits provided by each mode.4–10 One review10 evaluated the difference between aerobic and resistance exercises in this population; however, the inclusion criteria allowed the entry of papers that used insulin therapy. This fact may have introduced unreliability in the result, as the medication interferes with the patient's response to exercise not only by promoting normalization of glycemic levels but also of all metabolic aspects of diabetes.
Given the above, this systematic review aims to evaluate, through the GRADE system,11 the quality of the evidence of the published clinical trials investigating the effectiveness of resistance exercise when compared to aerobic exercise on clinical and metabolic outcomes in adults with type 2 DM who did not use insulin therapy during the studies. In addition, this review aims to investigate the prescribed exercise protocols with respect to frequency, intensity, and duration to guide evidence-based practice.
MethodsData sources and searchesFor this systematic review, searches were conducted in the electronic databases MEDLINE/PubMed (1966 – April/2016), CINAHL (1981 – April/2016), SPORTDiscus (1985 – April/2016), LILACS (1986 – April/2016), and SciELO (1998 – April/2016). There were no restrictions to language or time of publication, and the search strategies for each database (presented on the Table of Appendix A) took into account their specific descriptors through the Medical Subject Headings (MeSH), CINAHL Subject Headings, and Health Sciences Descriptors (DeCS). In addition, we screened from the reference lists of the eligible trials.
Study selectionThe articles selected for a more specific analysis included randomized controlled clinical trials published as full papers, studies that involved patients with type 2 DM over 19 years old, and articles which compared resistance exercise against aerobic exercise through a structured protocol with detailed description of both modalities.
The following were considered as primary outcomes: long-term glycemic control assessed by glycated hemoglobin (HbA1c) or short-term glycemic control assessed through postprandial or fasting blood glucose (FBG) concentration; cardiorespiratory fitness through maximal oxygen consumption (VO2max); and body mass index (BMI). As secondary outcomes, we considered blood lipid profile [total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglycerides] and the presence of adverse effects such as episodes of hypoglycemia, muscle fatigue, and mortality.
The following types of documents were excluded: letters, editorials, extended abstracts, studies that included patients with ulceration, skin lesions and/or rheumatic disease, the presence of chronic conditions, as well as patients with gestational diabetes and type 1 DM. Studies which involved prior or on-going insulin therapy, change in the use of corticosteroids, oral hypoglycemic drugs, or any kind of hypoglycemic diet two months before the start of the exercise protocol were also excluded.
Data extraction and quality assessmentThe searches, data collection, and content analysis of the selected studies were performed by two independent reviewers (CSN and KA), and the differences were discussed by a third evaluator (SRAM) using an eligibility criteria of a previously established protocol for the elaboration of the systematic review.
The quality of evidence for the outcomes HbA1, VO2max, BMI, total cholesterol, HDL, LDL, and triglycerides was assessed using the GRADE system and presented through the Summary of Findings Table (Table 1). The GRADE proposal classifies the level of evidence as high, moderate, low, or very low considering five factors that can affect the quality of the evidence of the outcomes of a clinical trial: design, risk of bias, inconsistency, indirectness, and imprecision.11 A review of the evidence for each factor followed the subsequent classification: no (no reduction in points), serious (reduction of 1 point), and very serious (reduction of 2 points),12 being scored by reviewers according to the interference biases detected in these items.
Summary of findings.
| Resistance exercise compared to aerobic exercise for type 2 diabetes mellitus |
| Patient or population: Type 2 diabetes mellitus |
| Setting: Resistance exercise compared to aerobic without insulin therapy in patients with type 2 diabetes mellitus |
| Intervention: Resistance exercise |
| Comparison: Aerobic exercise |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with aerobic exercise | Risk with resistance exercise | |||||
| HbA1c | The mean hbA1c was 0 | The mean hbA1c in the intervention group was 0.1 higher (0.15 lower to 0.34 higher) | – | 276 (7 RCTs) | ⊕○○○ VERY LOWa,b,c,d | |
| VO2max | The mean VO2max was 0 | The mean VO2max in the intervention group was 2.86 lower (3.9 lower to 1.81 lower) | – | 247 (5 RCTs) | ⊕○○○ VERY LOWb,c,e,f | |
| BMI | The mean BMI was 0 | The mean BMI in the intervention group was 0.75 higher (0.2 lower to 1.69 higher) | – | 293 (7 RCTs) | ⊕○○○ VERY LOWa,b,c,g | |
| HDL | The mean HDL was 0 SD | The mean HDL in the intervention group was 0.25 SD lower (0.49 lower to 0) | – | 267 (6 RCTs) | ⊕○○○ VERY LOWc,g,h,i | |
| LDL | The mean LDL was 0 | The mean LDL in the intervention group was 2.71 lower (13.15 lower to 7.73 higher) | – | 248 (5 RCTs) | ⊕○○○ VERY LOWb,c,g,j | |
| Triglycerides | The mean triglycerides was 0 | The mean triglycerides in the intervention group was 2.37 higher (12.96 lower to 17.7 higher) | – | 267 (6 RCTs) | ⊕○○○ VERY LOWc,d,k | |
| Total cholesterol | The mean total cholesterol was 0 | The mean total Cholesterol in the intervention group was 4.43 lower (14.74 lower to 5.87 higher) | – | 243 (5 RCTs) | ⊕○○○ VERY LOWb,c,g,l | |
| GRADE Working Group grades of evidence | ||||||
| High quality: We are very confident that the true effect lies close to that of the estimate of the effect | ||||||
| Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different | ||||||
| Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect | ||||||
| Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
CI, confidence interval; MD, mean difference.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
The randomization was not described in four studies. The allocation concealment was not clear in five studies. There was no blinding for the outcome assessment in four studies, no blinding of participants and personnel in five studies, and incomplete outcome data in two studies. Final decision: we rated down one level for study limitation.
Although homogeneity is observed in the statistical tests (p>0.05 and I2=0%), the exercise protocols differ between the studies. Final decision: we rated down one level for study limitation.
The outcome is considered substitute. Final decision: we rated down one level for study limitation.
The confidence interval reached the null effect. Final decision: we rated down one level for study limitation.
The randomization was not described in two studies. The allocation concealment was not clear in three studies. There was no blinding for the outcome assessment in two studies, no blinding of participants and personnel in five studies, and incomplete outcome data in two studies. Final decision: we rated down one level for study limitation.
The sample size did not reach the optimal information size and the confidence interval did not reach the null effect. Final decision: we rated down one level for study limitation.
The sample size did not reach the optimal information size and the confidence interval reached the null effect. Final decision: we rated down two levels for study limitation.
The randomization was not described in three studies. The allocation concealment was not clear in four studies. There was no blinding for the outcome assessment in three studies, no blinding of participants and personnel in four studies, and incomplete outcome data in one study. Final decision: we rated down one level for study limitation.
The heterogeneity index was 57% and the exercise protocols differed between the studies. Final decision: we rated down two levels for study limitation.
The randomization was not described in two studies. The allocation concealment was not clear in three studies. There was no blinding for the outcome assessment in two studies, no blinding of participants and personnel in three studies, and incomplete outcome data in one study. Final decision: we rated down one level for study limitation.
The randomization was not described in three studies. The allocation concealment was not clear in four studies. There was no blinding for the outcome assessment in three studies, no blinding of participants and personnel in three studies, and incomplete outcome data in one study. Final decision: we rated down one level for study limitation.
The randomization was not described in two studies. The allocation concealment was not clear in three studies. There was no blinding for the outcome assessment in two studies, no blinding of participants and personnel in three studies, and incomplete outcome data in one study. Final decision: we rated down one level for study limitation.
For the specific GRADE item ‘risk of bias’, the Cochrane Collaboration's risk of bias tool was used, which takes into consideration the items: randomization, allocation concealment, blinding, loss to follow up, selective outcome reporting, and early stopping of trials. The established guidelines to assess the risk of bias were high, low, or unclear.13 Risk was considered high when the item was not fulfilled, when the method by which the item was carried out was not reported, or when the method was not valid.14 Low risk of bias was considered when the item was fulfilled and reported inadequately,15 and unclear risk of bias when the available information was insufficient for a high or low risk of bias account is made, when information about the conduct was sufficient, but the risk of bias was really unknown, or when the analyzed item did not apply to the study in question.16
Data synthesis and analysisThe statistical analysis was performed using the Review Manager Software (RevMan) version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The data were typed independently by two reviewers and subsequently compared. A meta-analysis was performed of all primary outcomes and most of the pre-defined secondary ones. A subgroup analysis of the studies was carried out according to the duration of the protocol, from eight to 20 weeks and 21–48 weeks. The homogeneity of the studies was determined through the test of heterogeneity. Studies were considered homogeneous when p assumed a value greater than 0.05 and the heterogeneity index (I2) was classified as low (heterogeneity values up to 30%). A meta-analysis of random effect was used considering that the studies were not functionally equivalent and that the effect size varied across populations.
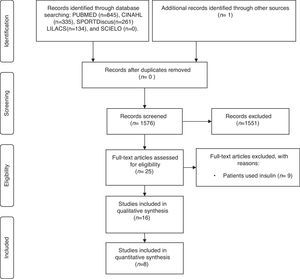
ResultsOf the 1576 articles found, 25 were selected for full-text evaluation and of those only 16 clinical trials17–32 were eligible for review (Fig. 1).
One article17 belonging to a group of clinical trials called Diabetes Aerobic and Resistance Exercise (DARE) yielded five publications,19,21,23,24,26 which reported results of different parameters. The same was observed between the studies of Arora et al.18 and Shenoy et al.,20 in studies by Jorge et al.25 and De Oliveira et al.,29 and between those of Bacchi et al.28 and Bacchi et al.32 As they used basal data from the same sample and the same methodology, these publications were considered in the review as results of the articles that originated them.17,18,25,28 Thus, of the 16 papers published, only eight17,18,22,25,27,28,30,31 studies are cited in the final review.
Description of studiesA total of 336 diabetic patients of both genders were evaluated. The studies were performed in Brazil, Canada, South Korea, Greece, India, Iran, Italy, and the USA. The predominant ethnicity was non-Hispanic white. The studies involved participants with an average age per group between 48 and 58 years and time of diagnosis of diabetes of one year or longer. The main criteria for inclusion consisted of sedentary individuals with DM type 2 for more than six months and who presented HbA1c between 6.5 and 11%. The reasons for exclusion and more details about the features of the studies are arranged in Table 2.
Characteristics of studies eligible for review in chronological order.
| Study, Year | Country | Sample Men:women (ER/EA) | Age (years) | Time of diabetes (years) | Inclusion criteria | Exclusion criteria |
|---|---|---|---|---|---|---|
| Arora et al.18, 2009 Shenoy et al.20, 2009 | India | 10 Men:10 Women (10/10) | GPR: 49.6±5.2 GA: 52.2±9.3 | GPR: 5.4±1.5 GA: 4.7±1.7 | Patients with type 2 diabetes for more than 6 months; Sedentary; not having performed any kind of strength training during the last year; not making use of insulin, men or women aged 40–70 years. | Individuals with subjective or objective evidence of coronary artery disease; uncontrolled hypertension; advanced neuropathy or retinopathy; serious orthopedic/cardiovascular/respiratory conditions that restrict physical activity. |
| Bacchi et al.28, 2012 Bacchi et al.32, 2013 | Italy | 28 Men:12 Women (20/20) | GR: 55.6±1.7 GA: 57.2±1.6 | GR: 9.7±1.7 GA: 10.7±1.4 | Patients with type 2 diabetes for at least 1 year; aged between 40 and 70 years, HbA1c between 6.5 and 9.0%, and BMI between 24 and 36kg/m2. Subjects had to be untrained on the basis of physical activity, 1000 MET min per week by the International Physical Activity Questionnaire (IPAQ). Weight had to remain stable for about 2 months before the program. | Individuals with moderate to severe somatic or autonomous neuropathy, cardiovascular disease, proliferative or pre-proliferative retinopathy and chronic kidney failure, receiving therapy with beta-blockers, smokers and those who are unable to execute the training protocols. |
| Jorge et al.25, 2011; de Oliveira et al.29, 2012 | Brazil | 10 Men:14 Women (12/12) | GR: 54.1±8.9 GA: 52.1±8.7 | GR: 7.7±3.9 GA: 5.45±4.1 | BMI between 25 and 40kg/m2; age between 30 and 70 years. | Individuals on insulin therapy; with type 1 diabetes, recent infections, liver or kidney failure, muscle or joint disability, active coronary artery disease, hypertension (>160/100mm Hg), heart failure, and a BMI>35kg/m2, using corticosteroids or hormone replacement therapy or supplementation with antioxidants or conditions that impede the practice of physical activity. |
| Kadoglou et al.30, 2012 | Greece | 13 Men:31 Women (23/21) | GR: 56.1±5.3 GA: 58.3±5.4 | GR: 7±2.9 GA: 7.6±2.4 | Overweight or obese individuals (BMI≥25kg/m2), with a diagnosis of diabetes >1 year and HbA1c levels [≥48mmol/mol (≥6.5%)]. | Individuals with inflammatory conditions, autoimmune diseases, hypothyroidism, osteoporosis, liver failure, kidney failure (creatinine >2.0mg/dl), diagnosed cardiovascular disease. Use of intense physical activity >2h/week. |
| Ku et al.22, 2010 | Korea | 28 Women (13/15) | GR: 55.7±6.2 GA: 55.7±7.0 | GR: 5.7±4.8 GA: 6.6±5.3 | Overweight women (>23kg/m2) with type 2 diabetes assisted at the Diabetes Center of Eulji Hospital, Seoul, Republic of Korea. | Individuals on insulin therapy; using lipid-lowering medications or thiazolidinedione; kidney, vascular, and liver complications; orthopedic problems. |
| Moe et al.27, 2011 | USA | 26 Men (13/13) | GR: 57.8±7.8 GA: 56.2±8.3 | GR: 4.8±4.2 GA: 4.7±3.8 | Men with type 2 diabetes, with no anti-diabetic medication other than metformin. | Presence of symptomatic cardiovascular disease, uncontrolled hypertension and physical or cognitive impairment. Individuals who regularly participated in intensive exercise during the 6 months before the beginning of the protocol. |
| Sigal et al.17, 2007; Jennings et al.19, 2009; Gavin et al.21,2010; Larose et al.23,2010; Reid et al.24,2010; Larose et al.26, 2011 | Canada | 79 Men:45 Women (64/60) | GR: 54.7±7.5 GA: 53.9±6.6 | GR: 6.1±4.7 GA: 5.1±3.5 | Patients with type 2 diabetes for more than 6 months; HbA1c with values of 6.6–9.9%; age between 39 and 70 years. | Individuals on insulin therapy; practicing 20min or more of exercise two or more times per week or some resistance training in the last six months; changes in the last two months of oral hypoglycemic, antihypertensive, or lipid-lowering medication or changes in body weight (≥5%); serum creatinine of 200μmol/l or more (≥2.26mg/dl); proteinuria greater than 1g/d; blood pressure greater than 160/95mmHg; restriction of physical activity from diseases; presence of another medical condition that makes participation impractical. |
| Yavari et al.31, 2012 | Iran | 30 all (15/15) | GR: 51.5±6.3 GA: 48.2±9.2 | >1 | DM2 established for more than one year; using oral hypoglycemic agents (not insulin); previous inactive lifestyle; level of A1c<11%. | Individuals with BMI 43, age of more than 70 years, with severe retinopathy, nephropathy and neuropathy, history of severe cerebrovascular or cardiovascular disease and serious musculoskeletal problems restricting physical activity. |
In relation to the aerobic exercises, walking was the predominant form, followed by exercise on a bike ergometer and cycle ergometer. While in the resistance exercises, the most widely used was resistance training of the major muscle groups. The protocols ranged from eight to 48 weeks lasting 20–60min/day, two to five times per week (Table 3). Of the eight studies included, one17 (published in19,21,23,26) did not report comparisons between resistance and aerobic groups.
Characteristics of exercise protocols.
| Study | Type of exercise | Pre-training phase | Protocol | Comments |
|---|---|---|---|---|
| Arora et al.18 Shenoy et al.20 | Aerobic | __________ | Walking exercise Frequency: three times a week Duration of session: 30min Duration of protocol: eight weeks | Did not specify the speed of the walk and exercise intensity. |
| Progressive resistance | __________ | 7 resistance exercises for the major muscle groups/session Frequency: twice per week 3 sets of 10 repetitions/exercise Initial intensity: 60% of RM 1 progressing to up to 100% of 1 RM Duration of protocol: eight weeks | Did not specify the interval time between sets; Did not cite the progression of intensity of exercises throughout the program. | |
| Bacchi et al.28 Bacchi et al.32 | Aerobic | __________ | Cardiovascular training equipment Frequency: three times a week Session duration: 60min Intensity: 60–65% FC Duration of protocol: four months | Did not specify the interval between sets. |
| Resistance | __________ | Nine resistance exercises for the major muscle groups/session; Three sets of ten repetitions Frequency of three times per week Session duration: 60min Intensity: 30–80% of 1 RM test Duration of protocol: four months | ||
| Jorge et al.25 de Oliveira et al.29 | Aerobic | __________ | Exercise bike Frequency: three times per week Session duration: 60min Intensity: HR corresponding to lactate threshold Duration of protocol: 12 weeks | Did not specify the intensity of the exercise; did not specify number of sets for each exercise, number of repetitions per set or interval between sets. |
| Resistance | __________ | Seven resistance exercises for the major muscle groups Frequency of three times per week Session duration: 60min Duration of protocol: 12 weeks | ||
| Kadoglou et al.30 | Aerobic | __________ | Exercise of walking, jogging on a treadmill, biking or gymnastics Frequency: four times week Intensity: 60–75% HRmax Session duration: 60min Duration of protocol: six months | Did not mention the interval time between sets. |
| Resistance | __________ | Eight different resistance exercises Two to three sets of eight to ten repetitions/session Frequency: 4 times per week Intensity: 60–80% of 1 RM Duration of session: up to 60min Duration of protocol: six months | ||
| Ku et al.22 | Aerobic | __________ | Walking exercise Frequency: five times per week Session duration: 60min Intensity: moderate (3.6–5.2 METs) Duration of protocol: 12 weeks | Did not mention the interval time between sets. |
| Resistance | __________ | Ten different resistance exercises Three sets of 15–20 repetitions Resistance exercises with elastic bands at 40–50% maximum capacity Frequency: five times per week (three times performed at the hospital gym and twice at home) Duration of protocol: 12 weeks | ||
| Moe et al.27 | Aerobic | Cycle ergometer exercise; Frequency: three times per week; Until being able to exercise for 45min at 75% of baseline of VO2max | Exercise on the cycle ergometer Frequency: two to five times per week Session duration: 45min Intensity: 50–85% of baseline of VO2max Duration of protocol: 12 weeks | |
| Resistance | __________ | Resistance exercises in five machines Three sets of eight repetitions (one to three min between sets)/machine Frequency: 5 times per week (three times performed at the hospital gym and twice at home) Intensity: 60% baseline 1–2.5kg Duration of protocol: 12 weeks | ||
| Sigal et al.17; Jennings et al.19; Gavin et al.21; Larose et al.23; Reid et al.24; Larose et al.26. | Aerobic | Aerobic exercise on a treadmill or cycle ergometer 15 to 20min/session Intensity of 60% HRmax Frequency of three times per week Duration of four weeks | Aerobic exercise on a treadmill or cycle ergometer Frequency: three times per week Session duration: 45min Intensity of 75% of HRmax Duration of protocol: 22 weeks | |
| Resistance | Seven resistance exercises performed on machines In the first two weeks – one sets of each exercise In the 3rd and 4th weeks – two sets of each exercise Frequency of twice per week | Seven resistance exercises performed on machines Three sets for each exercise Frequency: twice per week The amount of load for each exercise increased while the number of reps decreased to a maximum of eight repetitions Duration of a single exercise set: from 30 to 60s, with an interval of 2–3min between sets Session duration: 45min with 15–20min of active exercise Duration of protocol: 22 weeks | ||
| Yavari et al.31 | Aerobic | __________ | Exercise on a treadmill, elliptical machine, or exercise bike Frequency: two to three times per week Duration of the session: 20–60min Intensity: 60–75% HRmax Duration of protocol: 12 months | |
| Resistance | __________ | Ten resistance exercises for upper limbs and lower limbs Three sets of eight to ten repetitions/session 90–120s interval between sets Frequency: two to three times per week Intensity: 60–80% of 1 RM Session duration: 60min Duration of protocol: 12 months | ||
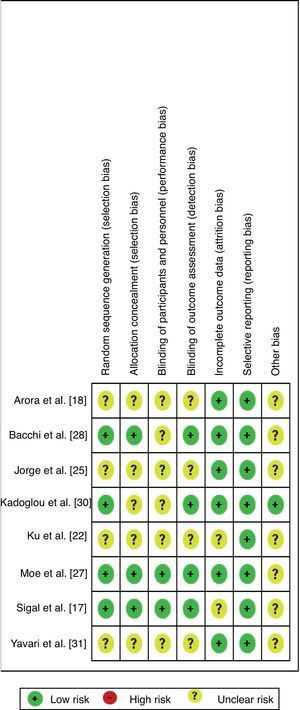
Four studies showed increased risk of bias18,22,25,31 for not describing the process of randomization, allocation, and if there was blinding of participants, trainers, and assessors (presented in the Figure of Appendix A). Articles related to the same group as represented by (1) Arora et al.18 and Shenoy et al.20; (2) Bacchi et al.28 and Bacchi et al.32; (3) Jorge et al.25 and De Oliveira et al.29; and (4) Sigal et al.17 (in the following publications19,21,23,24,26) only differed with regard to the bias of incomplete results and selective description.
Assessor blinding was conducted in only four studies17,27,28,30 (in five publications19,21,23,24,26). As for the bias of incomplete data, two articles17,22 (in one publication26) did not describe whether there were losses or exclusions. However, those that did report incomplete data described them mostly as a result of lack of attendance, presence of negative results in laboratory and/or diagnostic tests, time constraints, or personal reasons not favoring the risk of incomplete data bias. None of the authors described that they performed an intention-to-treat analysis, and this was not observed in the articles. Another prominent point refers to the sample size calculation, which was not performed in any of the included studies.
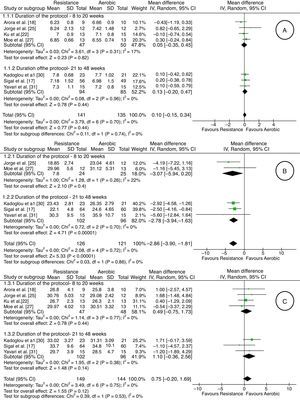
Measurement of outcomesIt was possible to perform five meta-analyses involving the outcomes HbA1c, VO2max, BMI, HDL, LDL, triglycerides, and total cholesterol, according to subgroups (eight to 20 weeks and 21–48 weeks) proposed in the Methods section. In relation to glycated hemoglobin (HbA1c), there was very low-quality evidence (downgraded due to risk of bias, inconsistency, indirectness, and imprecision) of lack of difference between the aerobic and resistance groups in both subgroups [seven studies17,18,22,25,27,30,31; 276 adults; difference in average % of 0.10; 95% CI: −0.15 to 0.34; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final values for each group with the confidence interval (aerobic group: MD: −0.4; 95% CI: −0.61 to −0.18; resistance group: MD: −0.35; 95% CI: −0.59 to −0.10).
Regarding VO2max, there was very low quality evidence (downgraded due to risk of bias, inconsistency, indirectness, and imprecision) of an increase of 2.86 (ml/kgmin) in favor of resistance exercise [five studies17,25,27,30,31; 247 adults; average difference in ml/kgmin of −2.86; 95% CI: −3.90 to −1.81; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final for each group with the confidence interval (aerobic group: MD: 4.0; 95% CI: 2.7–5.3; resistance group: MD: 2.1; 95% CI: 0.6–3.5).
There was no difference in relation to BMI between the aerobic and resistance groups, from very low-quality evidence (downgraded due to risk of bias, inconsistency, indirectness, and imprecision) [seven studies17,18,22,25,27,30,31; 293 adults; average difference in kg/m2 0.75; 95% CI: −0.20 to 1.69; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final values for each group with the confidence interval (aerobic group: MD: −0.76; 95% CI: −1.1 to −0.4; resistance group: MD: −0.54; 95% CI: −0.85 to −0.22).
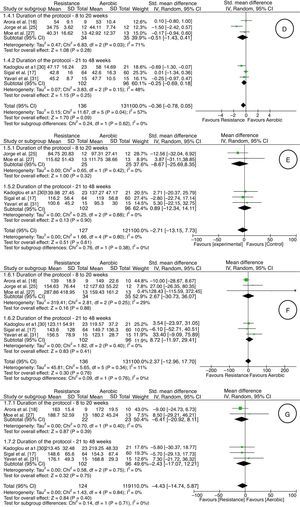
HDL values did not differ between the aerobic and resistance groups, from very low-quality evidence (downgraded due to risk of bias, inconsistency, indirectness, and imprecision) [five studies17,18,25,27,30,31; 267 adults; average difference in ml/kgmin of −0.36; 95% CI: −0.78 to 0.05; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final values for each group with the confidence interval (aerobic group: MD: 2.9; 95% CI: −0.28 to 6.1; resistance group: MD: 1.3; 95% CI: −1.1 to 3.8).
With regard to LDL values, there was very low-quality evidence (downgraded due to risk of bias, inconsistency, indirectness, and imprecision) of no difference between aerobic and resistance groups [five studies17,25,27,30,31; 248 adults; average difference in mg/dl of −2.71; 95% CI: −13.15 to 7.73; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final values for each group with the confidence interval (aerobic group: MD: 1.8; 95% CI: −9.9 to 13.5; resistance group: MD: 2.3; 95% CI: −4.5 to 9.2).
The rate of triglycerides, from a very low-quality evidence (downgraded due to risk of bias, inconsistency, indirectness, and imprecision), did not differ between the aerobic and resistance groups [six studies17,18,25,27,30,31; 267 adults; average difference in mg/dl of 2.37; 95% CI: −12.96 to 17.70; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final for each group with the confidence interval (aerobic group: MD: −27.8; 95% CI: −57.5 to 1.7; resistance group: MD: −23.9; 95% CI: −49.5 to 1.6).
As for total cholesterol, there was very low-quality evidence (downgraded due to risk of bias, inconsistency, indirectness and imprecision) of no difference between the groups [five studies17,18,27,30,31; 243 adults; mean difference in mg/dl of −4.43; 95% CI: −14.74 to 5.87; random effect] (Fig. 2). One study28 showed the mean difference between the baseline and final values for each group with the confidence interval (aerobic group: MD: −0.8; 95% CI: −15.8 to 14.1; resistance group: MD: −0.7; 95% CI: −8.5 to 7.1).
For all the outcomes mentioned above, one study28 was not included in the meta-analysis because it reported the data by mean change and confidence interval. It was not possible to calculate the standard deviation of the final values because the sample size varied from the baseline.
Only five studies17,27,28,30,31 mentioned the presence of adverse effects during the design of the study. Sigal et al.17 did not report such effects. However, the others reported transient hypoglycemia during physical training, hypoglycemic events, musculoskeletal pain, and need for insulin therapy during the study. Only one of the studies17 reported that the data were not different between the groups (RR=0.94; 95% CI: 0.25–3.58).
DiscussionThis meta-analysis rated the quality of evidence of the outcomes according to the GRADE system. Therefore, from a very low quality of evidence, the results of this systematic review indicate that the evidence regarding the effectiveness of resistance exercise compared to aerobic without insulin therapy in patients with type 2 DM showed no difference in values of HbA1c, BMI, HDL, LDL, total cholesterol, and triglycerides. However, there was an increase in VO2max, favoring patients who performed resistance exercise.
This positive result for VO2 in the patients who did resistance exercise corroborates the hypothesis that variations in VO2 tend to be associated with the intensity of exercise.33 This is due to a series of circulatory adjustments in response to an increase in cardiac demand, which contributes to greater transport of O2 and an increase in its use at the level of the skeletal muscle.31,34 However, given that this evidence is from very low-quality evidence, the interpretation should be analyzed with caution.
It is worth pointing out that, in four studies,18,22,25,31 most of the items were compromised since no allocation concealment or masking was described, in addition to failing to perform adequate randomization, which can affect the findings.35 In addition, some studies showed attrition bias, which may also overestimate the treatment. Intention-to-treat analysis was not mentioned in any of the articles. Its presence maintains the similarity of the allocated groups, and its absence may overestimate the clinical effect of treatment.36 One of these studies22 reported in its conclusion that, during the process of randomization, patients with better insulin sensitivity tended to be assigned to the aerobic group, possibly influencing the results by methodological negligence that caused a selection bias.
On the other hand, in studies using exercise as the intervention, the blinding of participants and trainers is unfeasible, which characterizes a bias of conduction and execution, but it could be minimized by the blinding of the evaluators. However, this procedure was only carried out in four studies.17,27,28,30
It should be noted that, in addition to all the bias detected, only one study17 presented sample size calculation. Therefore, it is questionable whether there was enough sample power in the other studies to reveal differences between the results. However, this study17 mentioned that it was not designed to compare the aerobic and resistance groups and reported the need for a larger sample, which highlights the low sample power of this population.
Another point of emphasis is the pre-training stage established in the studies of the DARE group.17 As this group established a previous training of four weeks of aerobic exercise for all participants of the test, this may have promoted a pre-screening of participants, generating a selection bias. Because only participants with the best performance attributes were selected to enter the final study sample, the data may not reflect reliable results, making an extrapolation to clinical practice difficult.
In relation to the other outcomes, it would be expected that resistance exercise, when compared to aerobic exercise, would significantly lower HbA1c values, because resistance training causes a greater absorption of glucose at the cellular level, thereby reducing hyperglycemia37–39 and additionally an increase in the BMI values due to increased lean body mass.40 Furthermore, resistance exercise would be expected to lower the risk of abnormalities related to lipid profile through HDL, LDL, total cholesterol, and triglycerides that are associated with the metabolic syndrome.5,40 However, such results were not observed, possibly due to the bias found among the studies as mentioned above.
Regarding the protocols used in the studies, there was variation in some parameters, however most studies used a duration between eight to 20 and 21–48 weeks. The aerobic protocols included walking or cycling exercises at a frequency of three to five times per week with 30 sessions lasting 45 or 60min each. The resistance exercise protocols had a frequency of two, three or five times per week with 45- or 60-min sessions. Furthermore, the limited number of studies did not allow a sensitivity analysis or meta-regression to assess the possibility of dose-response effects of exercise.
ConclusionBased on very low-quality of evidence, resistance exercise, compared to aerobic exercise in protocols with duration longer than 12 weeks, appears to be effective in promoting an increase in VO2max in diabetic patients. However, the effectiveness of this type of exercise in glycemic control (HbA1c) and lipid profile (HDL, LDL, total cholesterol, triglycerides) is still inconclusive. It is expected that future trials in this area have an appropriate sample power, randomization and allocation concealment, and standardized protocols. Moreover, it is important to ascertain the possible adverse effects and monitor long-term post-training outcomes.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thanks the Program for the Restructuring and Expansion of Federal Universities (REUNI) for the scholarship.
Search strategies used for MEDLINE/Pubmed, CINAHL, SPORTDiscus, LILACS, SciELO.
| Databases | Search strategies |
|---|---|
| MEDLINE/Pubmed | ((((“Diabetes Mellitus”[Mesh]) AND “Exercise”[Mesh]) AND “Adult”[Mesh])) NOT (((“Rheumatic Diseases”[Mesh]) OR “Ulcer”[Mesh]) OR “Skin”[Mesh]) Filters activated: Clinical Trial, Humans. |
| CINAHL | “Diabetes Mellitus, Type 2” AND “Diabetes Mellitus” AND “Exercise” AND “Resistance Training” AND “Aerobic Exercises” AND “Human” NOT (“Rheumatic Diseases” OR “Ulcer” OR “Foot Ulcer” OR “Skin”) |
| LILACS and SciELO | “Diabetes Mellitus” AND “Exercise” AND “Adult” AND NOT (“Rheumatic diseases” OR “Ulcer” OR “Skin”) |
| SPORTDiscus | “Type 2 Diabetes” AND “Training” NOT (“Rheumatic diseases” OR “Ulcer” OR “Skin”) |