Individuals commonly experience age-related systemic decreases in skeletal muscle strength, physical function, and mobility, leading to falls and potential associated hip fractures.
ObjectiveTo evaluate whether intensive exercise can improve physical function, mobility, and independence in activities of daily living (ADL) and shorten the length of hospital stay in older adults after hip fracture surgery.
MethodsThis systematic review was conducted under the PRISMA guidelines. Searches were performed on January 5, 2022 in eight databases. Randomized controlled trials (RCTs) were included. The participants included older adults with hip fracture, and the intervention studied was intensive exercise. The outcomes were physical function, mobility, ADLs, and the length of hospital stay. Meta-analyses were conducted using RevMan 5.3.
ResultsFifteen studies were included in this review. After hip fracture surgery, intensive exercise improved participants’ physical function to a greater extent than regular or no exercise (standardized mean difference [SMD] = 0.74; 95% CI: 0.25, 1.23). Intensive exercise was particularly more effective for gait speed (SMD = 0.15, 95% CI: 0.01, 0.30), the timed up-and-go test results (mean difference [MD] = -4.34, 95%CI: -6.74, -1.94), balance (SMD =0.42, 95% CI: 0.38, 0.89), and ADLs (SMD = 0.55, 95% CI: 0.24, 0.87). The quality of the evidence was low due to risk of bias, inconsistency, and imprecision.
ConclusionsIntensive exercise early post-operation provides potential additional benefits compared to no or regular exercises on older adults after hip fracture surgery.
Statistical reports released by the World Health Organization show that the incidence of hip fractures in older men and women worldwide is 6% and 18%, respectively, and that the number of hip fractures worldwide is expected to increase to 4.5–6.3 million by 2050.1 Most older adults who sustain a hip fracture experience a permanent decrease in physical functioning. Only 40% of patients exhibit the same functional status as before the fracture, 20% need long-term care, and approximately 13% are completely disabled.2
In the last two decades, a number of studies have explored the effects of intensive exercise after surgery in older adults with hip fracture.3-7 However, the effectiveness of this type of training is debated. For example, some randomized controlled trials have indicated that compared with regular or no exercise, this type of exercise significantly improves physical function, the Timed Up-and-Go test result, patients’ reaction time, and the 6-min walk test result, and reduces the length of stay in a hospital.7-9 Conversely, some studies have suggested that there are no significant differences in physical function, gait speed, or the Timed Up-and-Go test result between intervention and control groups.5,9,10
A Cochrane systematic review reported that well-designed exercise programs can improve the physical condition of older adults after hip fracture surgery, but there is no clear evidence showing which type of rehabilitation is most effective with respect to mobility recovery.11 An updated Cochrane systematic review12 suggested that higher intensity and frequency of exercise tended to show stronger effects on important outcomes. Previous systematic reviews have reported that lower-limb progressive resistance exercise and balance training can improve patients’ physical functioning, gait performance, lower-limb strength, performance during tasks, and independence in activities of daily living (ADLs).13,14
To date, a systematic review examining the effectiveness of intensive exercise after hip fracture surgery, that includes search results from PubMed, Embase, Cumulative Index of Nursing and Allied Health Literature (CINAHL), Cochrane Library, Chinese Biomedical Literature database (CBM), China national knowledge infrastructure (CNKI), Wanfang, and China Science and Technology Journal Database (VIP), has not been reported. Therefore, we conducted this systematic review and meta-analysis to determine the effects of intensive exercise among older adults after hip fracture surgery.
MethodsThe Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines were followed.15
Search strategyA comprehensive electronic literature search of PubMed, Embase, CINAHL, Cochrane Library, and four Chinese databases (CNKI; CBM; Wanfang; and VIP) was performed by two authors (F. B. & M. M. L.) to identify relevant publications that were published before January 5, 2022. The gray literature in Baidu Academics and Google Academics was also searched. Additional relevant publications were identified by searching the reference lists of the identified publications and existing relevant systematic reviews. The search strategy was modified for each database. The full search strategies for all English and Chinese databases are available in the Supplementary material – Search strategies.
Inclusion criteriaTrials were selected according to the predetermined criteria based on:
ParticipantsThe study population included older adults who did not have acute neurological impairment, severe cardiovascular diseases, unstable chronic or terminal illnesses, major depression, severe cognitive impairment, or severe musculoskeletal impairment. The start of the intervention could be in the early post-operation (up to 3 months), subacute (from 3 to 6 months), or late-stage (from 6 months up to 7 years).
Intervention groupThe intensive exercise intervention was defined as a higher intensity and duration of exercise than regular or no exercise. Specifically, (1) the intensity was higher than 60% of the 1-repetition maximum, it included more than 3 sets, and 8 repetitions each set were performed; OR (2) the exercises were performed two to three times per week, and each session lasted more than 30 min16 ;OR (3) the frequency was a minimum of 5 days per week. Functional training including gait, transfers, balance, and ADLs were performed daily. Sessions should be between 30 and 60 min duration depending on patient tolerance.17
The types of intensive exercise included progressive resistance training, resistance training, weight-bearing, strength, endurance, balance, power, and aerobic training.
Control groupThe control group, which served as the comparator, performed the following forms of regular or no exercise: (1) sat or laid down and walked for a short duration using parallel bars or walking aids; (2) continued their usual lifestyle and maintained their pre-study level of physical activity.
Outcome measuresThe primary outcome was physical function, defined broadly as any measure of overall physical function, including the modified Iowa Level of Assistance score,18 Tinetti performance-oriented mobility assessment score,19 modified physical performance test score,20 Harris hip score,21 Physical Performance and Mobility Examination,22 12-item short-form questionnaire,23 or the medical outcomes of the 36-item short-form24 health survey. The secondary outcomes included (1) mobility (such as the fastest gait speed,25,26 the Timed Up-and-Go test result,27 6-min walk test result,28 and balance: as rated by the Berg Balance Scale,29 National Health and Ageing Trends Study,30 or a modified balance test31; (2) independence in ADLs, as rated by Barthel index32 or Nottingham extended activities of daily living scale33; and (3) the length of hospital stay.
Study designOnly randomized controlled trials (RCTs) where participants were randomized to an intervention or a control group were considered eligible for this systematic review. Quasi-randomized clinical trials and other types of studies were excluded.
Study selectionAll searched records were imported into EndNote X9 to eliminate duplicate publications. The two authors (F. B. & M. M. L.) worked independently to identify studies that met the inclusion criteria. Preliminary screening was performed by reading the titles and abstracts. To further evaluate the eligibility of potential publications, full- texts of articles were evaluated and any disagreements were discussed with the third author (Z. W. W.).
Data extractionThe following information was extracted from the eligible studies: authors, publication year, country, sample size, participants’ mean age, study settings, intervention start time, study period, intervention details (e.g., form, intensity, daily/weekly frequency, duration per session, and duration of exercise), follow-up times, main outcomes, and the outcome measures used.
Risk of bias assessmentThe quality of the included RCTs was evaluated using the approach recommended by the Cochrane Handbook for Systematic Reviews of Interventions.34 The seven recommended items include random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias for each item was categorized as ‘low risk’, ‘unclear’, or ‘high risk’. All included studies were assessed independently, and disagreements between the two reviewers were resolved by the third reviewer.
Certainty of the evidenceThe Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system was utilized to assess the overall certainty of the evidence.35 From an initial starting point of high-certainty evidence, the level of evidence was downgraded (to moderate, low, or very low) for each of the following: risk of bias, inconsistency of results, indirectness, imprecision, and publication bias.
For risk of bias, we downgraded the quality of evidence by one level if 25% or more of the participants in the comparison were from studies with high risk of bias defined as one or more criteria classified as high risk of bias in the study. For inconsistency of results, quality of evidence was downgraded by one level if by visual inspection the presence of wide variation of effect estimates was verified or the I2 test was greater than 50%. For indirectness, quality of evidence was downgraded by one level if more than 50% of participants varied from the population of interest (e.g. mixed populations or multiple disorders). For imprecision, we downgraded the quality of evidence by one level if the sample analyzed was <400 participants and downgraded by two levels if the sample analyzed was <200 participants. For publication bias, we downgraded by one level if publication bias was identified by visual inspection of funnel plots if more than 10 studies were included in the comparison.
Data synthesis and analysisThe standardized mean difference (SMD) with the 95% confidence interval (CI) was used when studies used different measurement tools, while the mean difference (MD) with the 95% CI was used when studies used the same measurement tool. The level of heterogeneity was evaluated by the I2 method, and a value of I2 > 50% was considered to indicate significant heterogeneity. A fixed-effects model was used to calculate the pooled effect size if the data were not significantly heterogeneous. Otherwise, a random-effects model was used. Publication bias was assessed by the visual inspection of a funnel plot.36 Sensitivity analyses were performed by excluding one study at a time to confirm the consistency of the findings. If the significance of the total or combined results changed when one study was excluded, the results were considered unstable. RevMan 5.3 provided by Cochrane Collaboration was used for all statistical calculations.
Subgroup analysis was performed to identify the time the start of the intensive training would be most beneficial for patients. In the meta-analysis, the intervention start time was defined as occurring during early post-operation (up to 3 months postoperatively); subacute (which usually included programs that started soon after the completion of standard physical therapy; from 3 months up to 6 months postoperatively); or late-stage rehabilitation (from 6 months up to 7 years after fracture).37
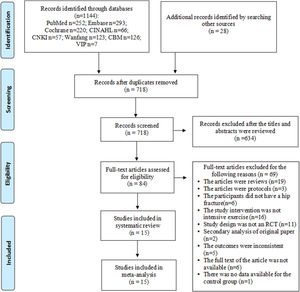
ResultsStudy selectionA total of 1172 records were retrieved by searching the databases and manually searching the reference lists. After 447 duplicates were removed, an additional 634 records were removed after the titles and abstracts were screened. In the end, 15 randomized controlled trials with a total of 1317 participants were included in the meta-analyses (Fig. 1).
Characteristics of included studiesFour studies were conducted in the United States, three studies were conducted in China, two studies each were conducted in Australia, Norway, and Finland, and one study each was performed in Denmark and Germany. Seven studies were conducted in a hospital, four studies took place in a hospital and at home, two studies were conducted in a specialized gym, one was conducted at home, and another was conducted in university research facilities.
The following eight types of intensive exercise were included: (1) resistance training, (2) weight bearing training, (3) strength training, (4) endurance training, (5) balance training, (6) power training, (7) progressive resistance training, and (8) aerobic training. Six studies4,9,10,38-40 were based on one form of intensive exercise, and nine5-8,10,41-44 were based on two or more forms of exercise.
For the control groups, the exercise types for 10 of the 15 studies5,6,8,38-40,42-45 could be summarized as follows: continued their usual lifestyle and maintained their pre-study level of physical activity. For the other five studies4,7,9,10,41 participants performed the following form of physical therapy: sat or laid down and walked for a short duration using parallel bars or walking aids.
Eight studies4,7,9,16,39,43-45 initiated the intervention during the early post-discharge rehabilitation (up to 3 months postoperatively), 3 studies8,10,40 initiated the intervention during the subacute rehabilitation (from 3 to 6 months postoperatively), and 4 studies5,6,38,40 initiated the intervention during late-stage rehabilitation (from 6 months up to 7 years postoperatively).
Detailed characteristics of each study are shown in Table 1.
Characteristics of the studies included in the systematic review.
| Study,year Country | Sample size (N) IG/CG | Mean ± SD age IG/CG | Settings | Intervention | Control | Follow-up | Main outcomes | Measurement tools | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention start time (post-operation) | Drop outs IG/CG | Form | Frequency/ duration | Dosage | Form | Frequency/duration | |||||||
| Mard et al.6 2008 Finland | 46 (24/22) | 74±6/ 74±7 | Senior gym | Within 6 months to 7 years | 1/2 | ①② | Twice a week/60–90 min per session | 60–80% of 1RM for the weaker leg and 50–70% of 1RM for the stronger leg | ② | Not mentioned | 12 weeks | ①⑤ | ①⑦ |
| Magaziner et al.41 2019 USA | 210 (105/105) | 80.3 ± 8.0/ 81.2 ± 8.8 | Home | 26 weeks | 9/4 | ③⑤⑧ | Twice-three times a week/>20 min per session | 3 sets of 8 repetitions per leg for each of 4 exercises | ① | 20 min per session | 40 weeks | ①②④⑥ | ①③⑩⑬⑯ |
| Portegijs et al.5 2008 Finland | 46 (24/22) | 73.8 ± 6.6/ 74.1 ± 7.2 | Senior gym | Within 6 months to 7 years | 3/2 | ①② | Twice a week/60–90 min per session | 60–80% of 1RM for the weaker leg | ② | Not mentioned | 12 weeks | ① | ① |
| Sylliaas et al.38 2012 Norway | 95 (48/47) | 82.4 ± 6.5/ 82.2 ± 5.1 | Hospital and home | 24 weeks | 3/2 | ① | Twice a week /45–60 min per session | Three sets of 10 repetitions at 80% of 1-RM | ② | Not mentioned | 12 weeks | ①②③④⑤⑦ | ①③⑤⑥⑦⑭⑰ |
| Kimmel et al.9 2016 Australia | 92 (46/46) | 81.3 ± 9.0/ 81.3 ± 7.5 | Hospital | Within 48 h of surgery | 0/0 | ① | 7 days per week/60 min per session | Not mentioned | ① | 7 days per week/30 min per session | 24 weeks | ⑤⑥⑦⑧ | ⑦⑧⑭ |
| Hauer et al.7 2002 Germany | 28 (15/13) | 81.7 ± 7.6/ 80.8 ± 7.0 | Hospital | After discharge | 3/1 | ③⑤⑦ | 3 days a week/>25 min per session | 70–90% of the individual maximal workload | ① | 3 days a week/60 min per session | 12 weeks | ①③④⑤⑥ | ①④⑦ ⑨⑰ |
| Lauridsen et al.44 2002 Denmark | 88 (44/44) | 60–89 /60–89 | Hospital | before discharge | 24/13 | ②③④ | Three times per week/ 120 min per session | Not mentioned | ② | 15–30 min per session/2 h per week | 15–22 days | ⑧ | |
| Guo et al.39 2019 China | 82 (41/41) | 72.19±5.42/72.96±5.84 | Hospital | First day after surgery | 0/0 | ⑦ | 7 days per week/25–50 min per session | 5 to 6 sets of 10–20 repetitions | ② | Not mentioned | 24 weeks | ⑥⑧ | ⑪ |
| Zhang et al.42 2017 China | 60 (30/30) | 67.43±2.81/68.27±3.38 | Hospital | First day after surgery | 0/0 | ③④ | Five days per week/ twice a day, 70 min per session | 5 sets | ② | 50 min per session/five days per week/twice a day | 12 weeks | ③⑥ | ③⑪ |
| Zhang et al.45 2019 China | 98 (49/49) | 77.48±2.32/77.51±2.31 | Hospital | First day after surgery | 0/0 | ⑦ | 7 days per week/20–60 min per session | 3 sets of 100–150 repetitions | ② | Not mentioned | 12 weeks | ③⑥ | ④⑪ |
| Moseley et al.4 2009 Australia | 160 (80/80) | 84±8/ 84±7 | Hospital | 14.71±9.06/13.41±7.55 d post-operation | 7/3 | ② | Twice daily/60 min per day | Repeating five weight-bearing exercises | ① | 30 min per day | 16 weeks | ①③⑥⑧ | ①④⑫ |
| Allegrante et al.43 2007 USA | 59 (32/27) | 78±7/ 77±8 | Hospital | Fourth or fifth week post-operation | 1/0 | ②③⑦ | Individualized retraining | 60% of 1-RM | ② | Not mentioned | 24 weeks | ① | ⑮ |
| Sylliaas et al.40 2011 Norway | 150 (100/50) | 82.1 ± 6.5/ 82.9 ± 5.8 | Hospital and home | 3months after a fracture | 5/7 | ① | Twice a week/45–60 min per session | Three sets of 10–15 repetitions of each exercise at 70% −80% of 1-RM | ② | Not mentioned | 12 weeks | ①②③④⑤⑦ | ①③⑤⑥⑦⑭⑰ |
| Mangione et al.8 2005 USA | 33 (11/12/10) | 77.9 ± 7.9/ 79.8 ± 5.6 | Arcadia University research facilities | 19.4 ± 11.7/ 19.7 ± 8.4/ 12.6 ± 2.3 weeks after surgery | 6/1/1 | ① | 1–2 times per week /30–40 min | 3 sets of 8 repetitions at the 80% of 1-RM | ② | Not mentioned | 12 weeks | ①②⑦ | ③⑮ |
| Peterson et al.10 2004 USA | 70 (38/32) | 79±7/ 78±8 | Hospital, home | 14±4weeks | 12/15 | ②③⑤ | Twice weekly/60 min per session | An individualized balance and gait training program | ① | Not mentioned | 26 weeks | ②⑤ | ③⑦ |
Note: 1-RM, one-repetition maximum; CG, control group; IG, intervention group. The 8-RM is strongly related to the 1-RM.37
Interventions: ①resistance training; ②weight bearing exercise; ③strength training; ④endurance training; ⑤balance training; ⑥power training; ⑦ progressive resistance training; ⑧ aerobic training.
Regular or no exercise include: ①sat or laid down and walked for a short duration using parallel bars or walking aids; ②continued their usual lifestyle and maintained their pre-study level of physical activity.
Outcomes: ①gait speed; ②6-min walk test (6MWT); ③independence in activities of daily living (ADLs); ④balance; ⑤Timed Up-and-Go test (TUG); ⑥mobility; ⑦physical function; ⑧length of stay in a hospital (LOS).
Measurements:①Ten-meter fast gait speed; ②50-ft fast walk; ③6 min walk test (6MWT); ④Barthel index; ⑤Berg Balance Scale (BBS); ⑥Nottingham Extended Activities of Daily Living scale (NEADL); ⑦TUG; ⑧modified Iowa Level of Assistance (mILOA) score; ⑨Tinetti performance-oriented mobility assessment (Tinetti's POMA); ⑩modified physical performance test (mPPT); ⑪Harris hip score; ⑫Physical Performance and Mobility Examination(PPME); ⑬National Health and Aging Trends Study (NHATS); ⑭12-item short-form questionnaire (SF-12); ⑮the medical outcomes of the 36-item short-form health survey (SF-36); ⑯a modified balance test; ⑰10-meter maximum walking speed test.
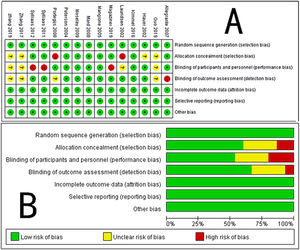
Six studies did not report details on allocation concealment, which could have caused selection bias. Four of the included RCTs were judged as having an ‘unclear’ risk of performance bias and detection bias because these processes were not reported adequately. All the included RCTs had complete datasets or reported the number of missing data points, and the reasons for the missing data were described in detail; therefore, the risk of attrition bias was judged as being ‘low’. There was no evidence of selective reporting bias or other bias in any of the included RCTs. An appraisal of the methodological quality of the included RCTs is shown in Fig. 2. The outcome measures and statistical analyses were appropriate for the type of research design selected.
GRADE was used to rate the certainty of the evidence. The overall evidence was low to moderate, which indicated that further research is likely to significantly change confidence in the effect estimate. The summary of findings is reported in Table 2.
Summary of findings and quality of evidence (GRADE) for intensive exercise.
| Certainty assessment | No. of participants | Effect size (95%CI) | Quality of evidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | No. of studies | Study design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication bias | Intervention | Control | ||
| Physical function | 11 | RCT | Not Serious | Serious | Not Serious | Not Serious | Undetected | 533 | 486 | SMD=0.74(0.25, 1.23) | ⊕⊕⊕⊝Moderateb |
| Mobility-gait speed | 8 | RCT | Serious | Not Serious | Not Serious | Not Serious | Undetected | 397 | 345 | SMD=0.15(0.01, 0.30) | ⊕⊕⊕⊝Moderatea |
| Mobility- Timed Up-and-Go test | 6 | RCT | Not Serious | Serious | Not Serious | Not Serious | Undetected | 268 | 209 | MD=−4.26(−6.64, −1.89) | ⊕⊕⊕⊝Moderateb |
| Mobility-balance | 3 | RCT | Serious | Not Serious | Not Serious | Serious | Undetected | 160 | 109 | SMD=0.64(0.38, 0.89) | ⊕⊕⊝⊝Lowa,c |
| Mobility-distance for the 6-MWT | 5 | RCT | Serious | Serious | Not Serious | Serious | Undetected | 302 | 244 | MD=40.80(−9.37, 90.96) | ⊕⊝⊝⊝Very lowa,b,c |
| Independence in ADL | 6 | RCT | Serious | Not Serious | Not Serious | Not Serious | Undetected | 312 | 265 | SMD=0.55(0.24, 0.87) | ⊕⊕⊕⊝Moderatea |
| Length of hospital stay | 3 | RCT | Not Serious | Serious | Not Serious | Serious | Undetected | 139 | 154 | MD=−3.05(−13.41, 7.31) | ⊕⊕⊝⊝Lowb,c |
Note: CI, confidence interval; MD, mean difference; RCT, randomized controlled trial; SMD, standardized mean difference.
Eleven studies evaluated the effectiveness of intensive exercise on physical function, as rated by the total score of the modified Iowa Level of Assistance,9 Tinetti performance-oriented mobility assessment,7 modified physical performance test,42 Performance and Mobility Examination,4 Harris hip score,16,39,45 12-item short-form questionnaire,38,40 and the medical outcomes of the 36-item short-form health survey.8,44 As shown in Fig. 3A, there is moderate certainty of evidence (downgraded due to inconsistency) that intensive exercise improved physical function (SMD =0.74, 95% CI: 0.25, 1.23, n = 1019, 11 trials, I2= 58.3%) compared to no or regular exercise group. Moreover, subgroup analyses showed that the effect of intensive exercise for the early post-operation group was significant (SMD =0.94, 95% CI: 0.20, 1.69, n = 565, 7 trials, I2= 94%), while no significant differences were found for the subacute rehabilitation subgroup (SMD = 0.07, 95% CI: −0.25, 0.39, n = 171, 2 trials, I2=96%) or late-stage rehabilitation subgroup (SMD = 0.61, 95% CI [−0.68, 1.90], n = 283, 2 trials, I2= 96%). However, there was a partial overlap in 95% CIs between early group and the other two groups.
Subgroup analysis was conducted on the basis of different intensive exercise based on load or duration. As shown in Fig. 3B, a significant difference was found for the effect of intensive exercise for the load group (SMD =0.61, 95% CI: 0.46, 0.76, n = 777, 9 trials, I2= 93%); no significant difference was found for the effect of intensive exercise on the duration group (SMD = −0.06, 95% CI: −0.31, 0.19, n = 242, 2 trials, I2= 52%).
Effect of intensive exercise on mobilityTen RCTs reported mobility outcomes, and 4 indicators were used to assess mobility: gait speed, the Timed Up-and-Go, balance, and the 6-min walk test (Supplementary material – Fig. 4).
Gait speed: Eight studies reported the effectiveness of intensive exercise on gait speed, as measured by the 10-meter maximum walking speed test,5-8,38,40 50-ft fast walk test,40 and 6-min walk test.8 There is moderate certainty evidence (downgraded due to risk of bias) that intensive exercise improved gait speed (SMD = 0.15, 95% CI: 0.01, 0.30, n = 742, 8 trials, I2= 0%) compared to no or regular exercise (Supplementary material – Fig. 4A).
Timed Up-and-Go test result: Six studies reported the effectiveness of intensive exercises on the time in seconds for the Timed Up-and-Go test.6,7,9,10,38,40 There is moderate certainty evidence (downgraded due to inconsistency) that intensive exercise reduced the time to complete the Timed Up-and-Go test (MD = −4.34 s, 95% CI: −6.74, −1.94, n = 477, 6 trials, I2= 80%) compared to no or regular exercise (Supplementary material – Fig. 4B).
Balance: Five studies reported the effects of intensive exercise on balance, but because the intervention components and duration conducted by Mangione et al42 were dramatically different from the other studies, this article was removed. Therefore, four studies were included in the meta-analysis, with the Berg Balance Scale,38,40 National Health and Ageing Trends Study,40 and a modified balance test,7 as outcome measures. There is low certainty evidence (downgraded due to risk of bias and imprecision) that intensive exercise improved balance (SMD =0.42, 95% CI: 0.38, 0.89, n = 269, 3 trials, I2= 0%) (Supplementary material – Fig. 4C) compared to no or regular exercise.
6-min walk test result: Five studies measured the effects of intensive exercise on the total distance in meters for the 6-min walk test.8,10,38,40,41 There is very low certainty evidence (downgraded due to risk of bias, inconsistency, and imprecision) that intensive exercise had no significant effect on the 6-min walk test result (MD = 40.80 m, 95% CI: −9.37, 90.96, n = 546, 5 trials, I2= 90%) (Supplementary material – Fig. 4D) compared to no or regular exercise.
Effect of intensive exercise on independenceSix studies reported the effectiveness of intensive exercise on independence in ADLs, as measured by the Barthel index4,7,42,45 and Nottingham extended activities of daily living.38,40 There is moderate certainty evidence (downgraded due to risk of bias) that intensive exercise improved independence (SMD = 0.55, 95% CI: 0.24, 0.87, n = 577, 6 trials, I2= 68%) (Supplementary material – Fig. 4E) compared to no or regular exercise.
Effect of intensive exercise on length of hospital stayThree studies4,9,44 measured the length of hospital stay. There is low certainty evidence (downgraded due to inconsistency and imprecision) that intensive exercise had no significant effect on length of hospital stay (MD = −3.05, 95% CI: −13.41, 7.31, n = 293, 3 trials, I2= 78%) (Supplementary material – Fig. 4F). The sensitivity analysis revealed that the findings were not significantly influenced by any single study.
Publication biasFor the meta-analysis of intensive exercise on physical function, there was no evidence of publication bias according to the inspection of the funnel plot.
DiscussionThis systematic review indicates that intensive exercise can better improve the physical function, mobility, and independence in ADLs of older adults after hip fracture surgery compared to when regular or no exercise are performed. The results of the subgroup meta-analysis suggest that intensive exercise may be effective when initiated early (up to 3 months) post-operation, which may not be the case for programs initiated later than 3 months post-surgery. The result is consistent with that in a previous study,46 and may due to the following mechanisms. After hip fractures, the occurrence of disuse atrophy increases, muscle mass decreases by 6% and fat content increases by 11%, half of which occurs in the first two months of the fracture, with a pronounced decrease in quadriceps muscle mass in the affected limb.47 According to Wolff's law,48 types of exercise impact bones differently and induce site-specific adaptations. Furthermore, some studies have recommended that the intensity of exercise for patients with hip fracture should be higher than normal levels to promote physiological adaptation (nerve supplementation or hypertrophy), which can result in training responses.49,50 Intensive exercise with more load showed greater improvements in muscle strength, balance, and functional ability.51 There were relatively few studies looking at different intervention start time, so the results that intensive exercise is effective only when started early should be interpreted cautiously, but does appear to make sense physiologically.
In the meta-analysis, intensive exercise was better than regular or no exercise for outcomes of mobility and independence in ADLs. For mobility, a significant difference was found for gait speed, the Timed Up-and-Go test, and balance ability, but not on the 6-min walk test. Magaziner et al.52 reported that gait was restored within 6–9 months postoperatively. In the meta-analysis, gait speed was measured 6 to 9 months after discharge, so the meta-analysis showed a small statistically significant difference; and the 6-min walk test distances were all measured 6 months or more after the hip fracture occurred, the participants showed the greatest improvement in gait performance, so no significant difference was shown. The intensive exercise group took less time to complete the Timed Up-and-Go test, which is important for predicting bone mineral density and fall risk, and this result is consistent with that in a previous study.53 Regarding the balance ability, sensitivity analysis shows a statistically significant effect of intensive exercise on balance when the study conducted by Mangione et al. is removed. After reading the article again, we found that the intervention components and duration of this study are quite heterogeneous from other studies.42 Therefore, this article was not included in the meta-analysis. For independence in ADLs, a RCT by Huusko et al.54 showed that greater independence in ADLs can be recovered in 3 months in a group of individuals undergoing intensive geriatric rehabilitation compared with a control group.55 One year later, there was no significant difference between the two groups. Therefore, patients should perform individualized intensive exercise early after hip fracture, as it can restore their ability to live independently as quickly as possible.
However, the intensive exercise and control groups showed no significant difference in the length of hospital stay. Perhaps there were no rehabilitation targets for discharging patients, there is a lack of uniform and clear discharge criteria, and there are differences in patient's disease characteristics and intervention methods.55 In this meta-analysis, there were two studies4,44 with high drop-out rates, mainly due to complications, dementia, and death. Disease characteristics also have an impact on the length of hospital stay.
LimitationsThere are some limitations of our systematic review. First, some RCTs did not describe the blinding and allocation concealment processes in detail, which may have affected the accuracy of the overall results. Second, in the inclusion criteria, older adults with moderate to severe cognitive impairment were not included in the study, conclusions about the training results may not be extended to these populations. Third, this review only included RCTs in English or Chinese. Finally, the review was not registered.
ConclusionIntensive exercise performed in older adults during the early post-operation stage with more load after hip fracture (up to 3 months postoperatively) have potential benefits and may improve physical function, mobility, and independence in ADLs more than when only regular or no exercises are done. It is suggested that individualized and supervised intensive exercise programs be performed in the early post-operation rehabilitation stage after surgery for hip fracture.
We want to thank Zhao Yajie and Xiao Hongmei of the Peking University School of Nursing for their help in evaluating the included trials.
This work was supported by the National Natural Science Foundation of China (No. 72274007).