The use of continuous positive airway pressure (CPAP) applied early after birth improves several outcomes when compared with intubation and invasive mechanical ventilation. “Early CPAP” protocols vary in relation to the pressure level, type of interface used, and studied sample.
ObjectiveThis study compared intubation rate, exogenous surfactant use, and hospitalization length (among other variables) prior to and after adopting an “early CPAP” protocol in preterm infants with gestational age between 28 and 32 weeks, using intermediate pressures and short binasal prongs.
MethodsThis was a retrospective study conducted in a public university hospital in Brazil. All preterm infants with gestational age between 28 and 32 weeks were included in the study. The newborns born between January 2011 and December 2012, prior to the protocol being implemented, were considered the historical control group, and those born after implementation, between February 2013 and August 2014 were considered the intervention group.
ResultsThe participants in both groups had similar baseline characteristics (p > 0.05). There were significant reductions in intubation rate (89% versus 73%, p = 0.02), exogenous surfactant use (86% versus 67%, p = 0.02), and median (Q1 - Q3) days of invasive mechanical ventilation [4 (2 - 14) versus 1 (0.15–9), p = 0.01] and length of hospital stay in days [56 (42–77) versus 42 (35–71), p = 0.02].
ConclusionsThe findings demonstrate positive outcomes of the early CPAP protocol. This protocol used simple and affordable equipment available in the hospital which could easily be reproduced in other centers, generating better outcomes for preterm infants and reducing hospital expenses.
Newborn respiratory distress syndrome (RDS) is one of the most frequent causes of respiratory failure and death among preterm infants.1 Invasive mechanical ventilation and surfactant administration were standard treatments for newborns with moderate or severe RDS for many years.1 However, invasive mechanical ventilation may damage the alveolar structure and is associated with pulmonary edema, inflammation, and fibrosis.1,2
The use of less invasive ventilatory support, such as continuous positive airway pressure (CPAP), reduces the intubation rate and the incidence of bronchopulmonary dysplasia (BPD) when applied early after birth when compared with intubation and invasive mechanical ventilation.3–5 Randomized clinical trials have observed reductions in surfactant use and days of mechanical ventilation when comparing an “early CPAP” group with an intubation group.6,7 The sample observed in most of these studies consisted of extreme preterm infants with gestational age less than or equal to 28 weeks. However, the use of this non-invasive ventilation modality could also benefit those born with gestational age between 28 and 32 weeks because surfactant production is still deficient in this period.8
“Early CPAP” protocols vary in relation to the pressure level and the type of interface used. Very high pressures such as 8 cmH2O may cause pneumothorax, while very low pressures such as 4 cmH2O may not be effective.6–9 Regarding interfaces, the literature indicates that short binasal prongs are more effective in preventing reintubation when compared with single and nasopharyngeal prongs.1–10 The “early CPAP protocol” has been used in different neonatal units.6,7 However, to the best of our knowledge, its use in public hospitals in Brazil has not been documented. Therefore, the objective of this study was to compare intubation rate, frequency of BPD, days of ventilatory support, oxygen therapy duration, surfactant use, days of hospital stay, and morbidities prior to and after adopting an “early CPAP” protocol in preterm infants with gestational age between 28 and 32 weeks, using intermediate pressures (6–7 cmH2O) and short binasal prongs in a public university hospital which serves a low-income population in Brazil. Knowledge gained from this study will hopefully contribute to better outcomes for preterm infants with gestational age between 28 and 32 weeks while reducing the costs for the health care system.
MethodsThis is a retrospective study with a historical control group conducted at a neonatal intensive care unit (NICU) and an intermediate care unit (IMCU) of a public hospital in Belo Horizonte, Minas Gerais, Brazil. A search was conducted among 156 records of preterm infants who were born between January 2011 and August 2014. The preterm infants born between January 2011 and December 2012 prior to implementing the “early CPAP” protocol was considered the historical control group, while those born between February 2013 and August 2014 after its implementation were considered the intervention group. All data were collected from historical records provided by the hospital after approval of the study by the Odilon Behrens Hospital research ethics committee (049/2013).
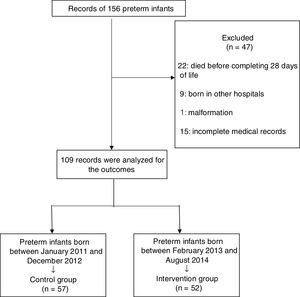
All preterm infants with gestational age between 28 and 32 weeks were included in the study. The exclusion criteria were newborns with apparent malformations or genetic syndromes; born in other hospitals, at home or in other places; those transferred and/or who died before the 28th day of life; and those with incomplete medical records.
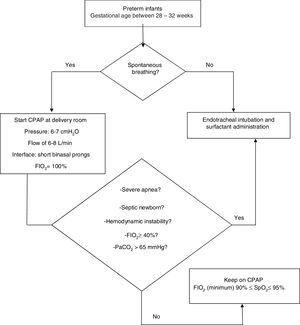
The “early CPAP” protocol was used in newborns with gestational age between 28 and 32 weeks, with suspicion of newborn RDS and spontaneous breathing and without a need for intubation for resuscitation. According to the protocol, newborns meeting these criteria were placed on non-invasive ventilation. An Inter® 3 mechanical ventilator (Intermed, São Paulo, Brazil) was used immediately after birth in CPAP mode with a pressure of 6–7 cmH2O for all newborns, with short binasal prongs, an inspired oxygen fraction (FIO2) of 100%, and a flow of 6−8 L/min. Then, these newborns were transported to the NICU/IMCU and their FIO2 were adjusted to guarantee a peripheral oxygen saturation (SpO2) between 90% and 95%. The CPAP suspension criterion was based on the clinical evaluation by health professionals, such as abdominal distension, oxygen need, respiratory distress, and nasal septum injury, among others. Endotracheal intubation and surfactant administration were considered in the following situations: partial pressure of arterial carbon dioxide (PaCO2) > 65 mmHg; FIO2 ≥ 40% to SpO2 ≥ 88%; hemodynamic instability, defined as low blood pressure for gestational age, poor perfusion, or both, requiring volume or pressure support for a period of 4 h or more; severe apnea, requiring positive pressure ventilation (self-inflating bag); or septic newborn.6,11,12Fig. 1 shows the “early CPAP” protocol.
The preterm infants in the historical control group received the following care soon after birth. The strategy for those with spontaneous breathing without important respiratory distress consisted of oxygen inhalation, or endotracheal intubation in the delivery room when apnea or important respiratory distress was present. The newborns were immediately sent to the NICU or IMCU after performing the procedures with only oxygen inhalation or intubated underwent positive pressure ventilation (self-inflating bag) and oxygen. Newborns who were intubated in both the historical control group and in the intervention group were ventilated based on clinical and arterial blood gas parameters. The parameters were changed according to the blood gas analysis, always trying to perform gentle ventilation and tolerating a PaCO2 of up to 65 mmHg. A flow of 6−8 L/min and a minimum FIO2 was used to guarantee a SpO2 between 90% and 95%.
An Inter® Neo mechanical ventilator (Intermed, São Paulo, Brazil) was also used in this study. CPAP was offered through an Inter® 3 or Inter® Neo upon arriving in the NICU/IMCU, depending on their availability. The ventilatory modes chosen were the same for both groups: intermittent mandatory ventilation, synchronized intermittent mandatory ventilation, and pressure-assisted/controlled ventilation, according to the infant’s need. The choice of the ventilation mode varied according to the clinical parameters and the ventilator availability in the NICU/IMCU. We used the “intermittent mandatory ventilation” mode when Inter®3 was available in intubated newborns; the “synchronized intermittent mandatory ventilation” mode when the newborn had minimal ventilation parameters, close to extubation; or the “pressure-assisted/controlled ventilation” mode when the Inter® Neo was available. Thus, the ventilation modes were chosen following this rationale in both the historical control and intervention groups. The newborns in the historical control group were intubated or received inhaled oxygen at birth, while newborns in the intervention group received CPAP (non-invasive ventilation mode), inhaled oxygen, or were intubated (if they did not have spontaneous breathing).
The following variables were used to characterize the groups: birth weight (grams), gestational age (weeks), sex, adequacy of birth weight for gestational age (small for gestational age (SGA) = neonates with weight below the 10th percentile; adequate for gestational age (AGA) = neonates with weight between the 10th and 90th percentiles), 1- and 5-minute Apgar scores, birth delivery type, and antenatal corticoid use. Regarding ventilatory assistance, the groups were characterized according to the first type of ventilatory support used by the newborn in the delivery room: oxygen inhalation, CPAP, or invasive mechanical ventilation.
The primary outcome to investigate the effect of protocol implementation was (1) intubation rate. The secondary outcomes were: (2) BPD frequency in each of the groups – BPD was defined as the need for continuous use of supplemental oxygen above 21% until the 28th day of life13; (3) days of use of each type of ventilatory support; (4) total oxygen therapy time; (5) use of exogenous surfactant; (6) length of hospital stay; and (7) complication rates of pneumothorax, periventricular-intraventricular hemorrhage, prematurity retinopathy, and necrotizing enterocolitis to describe morbidity.
Sample size calculationWe used 30 available medical records to estimate the sample size considering the variable intubation rate for calculation. The Chi-squared value found according to the calculations was 3.96. The value of the effect size (w) was 0.36. Thus, considering this effect size it would be necessary to collect a minimum of 81 medical records in total to reach a statistical power of at least 90% at a significance level of 0.05.14
Statistical analysisThe data distribution of the investigated variables was tested using the Kolmogorov-Smirnov test. The results were presented through descriptive statistics with frequency values for categorical variables and medians and interquartile ranges for non-normally distributed variables. Statistical analysis was performed using the Mann-Whitney test for comparison of medians and the Chi-square test to compare frequencies. A significance level of 5% was established. The analyses were performed in the Statistical Package for the Social Sciences® (SPSS, Chicago, IL, USA), version 17.0 for Windows.14
ResultsThe records of 156 newborns were analyzed, of which 47 were excluded: 22 newborns died prior to 28 days of life, nine were born in other hospitals, one was born with a malformation, and 15 had incomplete records. Thus, 109 records were analyzed. Fig. 2 shows the study flowchart.
Table 1 provides the characteristics of the infants included in the study. No significant differences were found between the study groups for any of the variables (p > 0.05).
Baseline characteristics between the study groups.
| Variable | Control group (n = 57) | Intervention group (n = 52) | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 27 (47) | 22 (42) | 0.59 | |
| Birth weight (grams) | 1210 | 1265 | 0.10 | |
| (985–1465) | (1042.5–1656.3) | |||
| Gestational age (weeks) | 29 | 30 | 0.10 | |
| (28−31) | (29−31) | |||
| SGA | 7 (12) | 4 (7.7) | 0.42 | |
| AGA | 50 (88) | 48 (92) | ||
| Apgar score at 1 min | ||||
| < 7 | 17 (30) | 16 (31) | 0.91 | |
| ≥7 | 40 (70) | 36 (69) | ||
| Apgar score at 5 min | ||||
| < 7 | 1 (1.8) | 1 (1.9) | ||
| ≥7 | 56 (98.2) | 51 (98.1) | 0.94 | |
| Type of delivery Cesarean section | 47 (82) | 37 (71) | 0.16 | |
| Use of antenatal corticosteroids | 33 (58) | 35 (67) | 0.31 |
Data are presented as absolute frequency and percentage (%) or median and interquartile range (IQR). SGA, small for gestational age; AGA, adequate for gestational age.
Regarding the type of ventilatory support used in the delivery room in the historical control group, most of the newborns were intubated (n = 37; 65%), while the remaining received oxygen inhalation (n = 20; 35%). The majority of newborns in the intervention group received “early CPAP” (n = 28; 54%), while five (10%) received oxygen inhalation, and 19 (36%) were intubated. Despite adopting the “early CPAP” protocol, it was not possible to control the action of all professionals. Thus, some newborns received oxygen and not CPAP as recommended in the protocol.
Table 2 shows the evaluated outcomes. Significant differences were observed in relation to intubation rate (p = 0.02), invasive mechanical ventilation days (p = 0.01), surfactant use (p = 0.02), and days of hospital stay (p = 0.02), which were all lower in the intervention group. There were no significant differences for the other variables (p > 0.05), and there were no significant differences between the groups regarding complications.
Outcomes and complications.
| Variables | Control group (n = 57) | Intervention group (n = 52) | p- value |
|---|---|---|---|
| Intubation rate | 51 (89) | 38 (73) | 0.02* |
| Bronchopulmonary dysplasia | 21 (37) | 17 (33) | 0.64 |
| Invasive mechanical ventilation (days) | 4 (2−14) | 2 (0.15−9) | 0.01* |
| Non-invasive ventilation (days) | 6 (3−11) | 5 (3−11) | 0.98 |
| Oxyhood (days) | 2 (0−4) | 1 (0−2) | 0.06 |
| Nasal catheter (days) | 0 (0−9) | 0 (0−13) | 0.47 |
| Duration of oxygen therapy (days) | 18 (7−46) | 12 (5−53) | 0.30 |
| Exogenous surfactant | |||
| Yes | 49 (86) | 35 (67) | 0.02* |
| No | 8 (14) | 17 (33) | |
| Length of hospital stay (days) | 56 (42−77) | 42 (35−71) | 0.02* |
| Complications | |||
| Pneumothorax | 1 (1.7) | 0 (0) | 0.33 |
| Periventricular-intraventricular hemorrhage | 4 (7) | 5 (9.6) | 0.62 |
| Retinopathy of prematurity | 10 (17) | 5 (9.6) | 0.23 |
| Necrotizing enterocolitis | 1 (1.7) | 1 (1.9) | 0.94 |
Data are presented as absolute frequency and percentage (%) or median and interquartile range (IQR). *p < 0.05.
A total of 40 newborns in the historical control group as well as 31 in the intervention group used antibiotics. In relation to infection, the historical control group had a significantly higher rate of sepsis compared to the intervention group (70.2% versus 46.2%, p = 0.01).
DiscussionSignificant reductions in intubation rate, exogenous surfactant use, days of invasive mechanical ventilation, and length of hospital stay were observed after implementing the “early CPAP” protocol in preterm infants with gestational age between 28 and 32 weeks using intermediate pressures (6–7 cmH2O) and short binasal prongs.
The results found in the present study are consistent with those previously reported in the literature. Three randomized controlled trials compared two intervention types in the delivery room: “early CPAP” versus conventional approach (intubation and surfactant use).6,7,15 Morley et al.6 studied preterm infants with gestational age between 25 weeks 0 days and 28 weeks 6 days. Those allocated to the “early CPAP” group received 8 cmH2O pressure and used either single or short binasal prongs. Finer et al.7 included preterm infants with gestational age between 24 weeks 0 days and 27 weeks 6 days in their sample and administered “early CPAP” via a T-piece resuscitator (5 cmH2O), but did not mention the type of interface used in the NICU. Dunn et al.15 studied preterm infants with gestational age between 26 weeks 0 days and 29 weeks 6 days using “early CPAP” of 5 cmH2O, which could be increased up to 7 cmH2O, in addition to short binasal prongs as the interface of choice. The authors of these studies observed that the “early CPAP” approach was able to reduce the intubation rates,7,15 surfactant use,6,15 and the invasive mechanical ventilation duration6,7 when started in the delivery room in extreme preterm infants.
A significant reduction in number of days in hospital stay was observed in favor of the CPAP group. Morley et al.,6 Miksch et al.,4 and Gittermann et al.5 also investigated this variable, but found no significant difference between the groups. It can be hypothesized that this difference is related to the fact that these studies included only extreme preterm infants. There were no significant differences between the groups for the other variables studied (i.e., days of using any ventilatory support and total oxygen therapy time). These results are consistent with the findings of the studies surveyed.4–7,15
Observational studies suggest that the “early CPAP” approach is associated with a lower incidence of BPD.3,4,16,17 In 1987, Avery et al.18 reported that the center which used CPAP as the initial respiratory support among the eight neonatal centers surveyed had a lower BPD rate compared with centers that used intubation and invasive mechanical ventilation as the initial approach. A retrospective study showed that the Columbia center, where the initial approach is “early CPAP”, continues to have a lower BPD rate than centers which prioritize mechanical ventilation and surfactant administration.18 However, there was no significant BPD reduction in the “early CPAP” group in the present study. Given this finding and because the beta value was high (0.83), the conclusion about this lack of difference is weakened.14 The sample size was likely insufficient for this variable, not allowing conclusions.
There were no significant differences between the groups regarding the pneumothorax, periventricular-intraventricular hemorrhage, prematurity retinopathy, or necrotizing enterocolitis rates. To our knowledge, only one study documented an increase in the pneumothorax rate in the “early CPAP” group in relation to the intubation group, perhaps due to the high CPAP pressure used.6 The findings related to these variables suggest that intermediate pressures may be sufficient to maintain airway pressurization without increasing the pneumothorax rate. We can hypothesize that there was an association between antibiotic therapy and the infection rate, as the infection rate and the intubation rate were higher in the historical control group, which may explain this result. It is well established that intubated patients are more susceptible to infection.
The “early CPAP” protocol implemented in this study used low-cost equipment available at the hospital and differed from the protocols of other studies in the following aspects: gestational age, CPAP pressure, and interface used.6,7,15 Most of the analyzed studies included extreme preterm infants (IG ≤ 28 weeks) in the sample, while the present study also included intermediate preterm infants, extending the age range to newborns with gestational age between 28 and 32 weeks because these infants could also benefit from positive pressure at birth.,6–8,15 There is no consensus on the ideal CPAP pressure range to be used for preterm infants.16 A recent study compared different pressure ranges with low pressure (4–6 cmH2O) versus high pressure (7–9 cmH2O) after extubation, showing that the extubation failure and reintubation rates were significantly lower in the high CPAP pressure group.19 In contrast, Morley et al.6 used a CPAP pressure of 8 cmH2O and observed a significant increase in the pneumothorax rate in the group that received CPAP in the delivery room compared with the group that was intubated. In view of this difference, the protocol in the present study used intermediate CPAP pressures (6–7 cmH2O) to ensure sufficient pressure to keep the alveoli open, but with a lower risk of pneumothorax. Studies show that short binasal prongs present less resistance to airflow and are more effective in preventing reintubation when compared with simple and nasopharyngeal prongs.20–22 Moreover, the results available in the literature are contradictory and inconsistent when nasal prongs are compared with nasal masks, not allowing to confirm if one approach is superior to the other. Literature shows that the nasal mask and the short binasal prong were effective in preventing intubation and that the nasal mask caused less nasal trauma. These authors suggest alternating the interfaces every 72 h of use because the interfaces cause nasal trauma in different regions.21,22 Thus, short binasal prongs were used in the present protocol based on these findings and the availability of interfaces in the hospital.
It is worth remembering that the study design (i.e. retrospective with a historical control group) has limitations regarding internal validity, such as a lack of control over the variables and the quality of the measures. As a randomized controlled trial was not carried out, we cannot say that the improvement in clinical outcomes is solely attributed to the implemented protocol. There may have been an improvement in both obstetric and neonatal care, among other factors. However, we controlled the devices (i.e. ventilators, short binasal prongs), which was the same in both periods, and the environment where the treatment of both groups was performed. In addition, we have no data regarding the specific number of children who used each ventilatory mode, nor the ventilator parameters used for each infant. Furthermore, 10% of the newborns received oxygen and not CPAP despite what is recommended in the protocol, which means that the protocol adherence was not total. Moreover, although our sample calculation was rigorous (with a desired power of 90% instead of the common 80%) and resulted in a large number of medical records, detection of significant differences was limited to the prior estimated effect size, and possible differences with smaller effect sizes may have not been detected (possible type 2 error). Finally, the data were obtained from available medical records, which does not exclude the possibility of error.
We have recently observed a change in the respiratory approach to preterm infants in the delivery room.23,24 Recent studies show that CPAP may be a good alternative to intubation and surfactant administration because it keeps the alveoli open during expiration, reduces respiratory work, improves functional residual capacity, and helps stabilize the rib cage.23 It reduces the need for therapy with exogenous surfactant and invasive mechanical ventilation when applied soon after birth.23,24 The protocol used simple equipment available at the hospital and can be easily reproduced in other centers, generating better outcomes for preterm infants and reducing hospital expenses.
ConclusionThe findings of the present study reinforce the benefit of using early CPAP (a current approach) and point to an innovative aspect, namely its use for preterm infants with gestational age between 28 and 32 weeks, in addition to using short binasal prongs as the interface of choice and intermediate CPAP levels. There were significant reductions in intubation rate, exogenous surfactant use, invasive mechanical ventilation days, and length of hospital stay after applying an early CPAP protocol in a public hospital.
Conflict of interestThe authors declare no conflicts of interest.
The work was partly financed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant 309990/2017-3.