Trigger point dry needling interventions are utilized by physical therapists to manage shoulder pain. Observational studies have shown positive short-term outcomes in patients with subacromial pain syndrome receiving trigger point dry needling. However, little research has been done to evaluate the long-term effectiveness of trigger point dry needling specifically as it compares to other commonly utilized interventions such as exercise and manual therapy. The purpose of this study is to assess the additive short and long-term effectiveness of trigger point dry needling to a standard physical therapy approach of manual therapy and exercise for patients with subacromial pain syndrome.
MethodsThis multicenter randomized trial with 3 arms was designed following the standard protocol items for randomized interventional trials. Results will be reported consistent with the consolidated standards of reporting trials guidelines. 130 participants will be randomized to receive standard PT interventions alone (manual therapy and exercise), standard PT and trigger point dry needling or standard PT and sham trigger point dry needling. The primary outcome measures will be the Shoulder Pain and Disability Index and Patient Reported Outcomes Measurement Information Systems (PROMIS-57) scores collected at baseline, 6-weeks, 6-months and one year. Healthcare utilization will be collected for 12 months following enrollment and groups analyzed for differences.
DiscussionIt is not known if trigger point dry needling provides long-term benefit for individuals with subacromial pain syndrome. This study will help determine if this intervention provides additive benefits over those observed with the commonly applied interventions of exercise and manual therapy.
Trial registrationIdentifier: NCT03442894 (https://clinicaltrials.gov/ct2/show/NCT03442894) on 22 February 2018.
Shoulder pain is a common musculoskeletal complaint, with point prevalence estimates ranging from 7% to 34%.1 Subacromial Pain Syndrome (SAPS), also known as subacromial impingement syndrome (SIS), is among the most common causes of shoulder symptoms and shoulder related disability.2 The term SAPS was coined because symptoms in individuals with SIS have not consistently been shown to arise from impingement of subacromial structures with the overlying acromion.3 SAPS is defined by Diercks et al.3 as “…shoulder problems that cause pain, localized around the acromion, often worsening during or subsequent to lifting of the arm”.3 Under this moniker, the diagnoses rotator cuff tendinopathy, subacromial bursitis, partial rotator cuff tears, biceps tendinopathy and calcific tendinitis are all classified as SAPS.3
Recently, trigger point dry needling (TPDN) interventions have begun to be utilized by physical therapists to manage musculoskeletal conditions to include shoulder pain.4 TPDN uses an acupuncture-like needle to target myofascial trigger points (MTrPs).4 MTrPs are defined as areas of hardness and tautness in muscles that contain hyperalgesic zones.4 Although there is disagreement on their pathophysiology, MTrPs have been reported to generate musculoskeletal symptoms including reduced range of motion (ROM) of surrounding joints, pain, altered joint mechanics and muscle fatigue.5 MTrPs have also been found to be associated with shoulder pain syndromes, including SAPS.6 Several observational studies have shown positive short-term outcomes in individuals with shoulder pain treated with TPDN.7 However, very little research has been done to evaluate the long-term effectiveness of TPDN, specifically as it compares to other commonly utilized interventions such as exercise and manual therapy.4
A recent clinical trial by Perez-Palomares et al.6 compared a program of manual therapy and exercise to the same program (manual therapy and exercise) and 3 TPDN sessions in individuals with SAPS.6 Subjects were followed for 3 months and received 10 visits of individualized physical therapy interventions (manual therapy and exercise). Half of the subjects also received 3 sessions of TPDN, targeting trigger points identified by the treating physical therapist. No differences in pain or shoulder function were observed out to the 3 months follow up. Arias-Buria et al.8 compared a 5-week program of exercise alone to the same program with 2 sessions of TPDN in individuals with SAPS.8 Patients in the TPDN plus exercise group showed greater improvement in shoulder disability, but not shoulder pain, at all follow-up points out to one year.8
Lewis and colleagues explored the value of adding acupuncture or electroacupuncture to a therapeutic exercise program in individuals with SAPS.9 Subjects were randomized into 3 groups, 6 visits of exercise alone, 6 visits of acupuncture and exercise and 6 visits of electro-acupuncture and exercise. They found no differences between groups for any of the outcomes assessed out to 12 months. Of note, there is disagreement among authors regarding whether acupuncture should be considered a distinct intervention from TPDN.10 However, in this study the acupuncture and electroacupuncture interventions were delivered by physical therapists and the needles were inserted locally in the area of shoulder symptoms intramuscularly, thus closely mimicking a typical dry needling intervention performed by a physical therapist.
While these studies have improved our understanding of the value of TPDN for patients with SAPS, the research on this topic to date has several limitations. First, several of the studies have only 2–3 sessions of TPDN over the course of the treatment period.6,8 Although there is no universally agreed upon dosage of TPDN, our clinical experience suggests TPDN is routinely delivered at a higher frequency over the course of an episode of care when treating individuals with SAPS. Another limitation of the research to date is the lack of a sham or placebo comparison group.6,8,9 The addition of a sham TPDN comparison group would help to determine if observed differences in clinical outcomes between groups were due to a treatment effect from the TPDN or to a placebo effect. Finally, to date, few studies have explored downstream healthcare utilization (HCU) after initial management of shoulder pain. Value-based care has been defined by Tsevat and Moriates11 as the ability to maximize the “outcomes achieved per dollar spent.”11 Therefore, in addition to collecting self-reported outcomes, capturing downstream medical care and total costs in the year following the intervention is an important variable for determining the comparative value of each intervention.
Considering the aforementioned shortcomings of previous research, the purpose of this study is to assess the additive short and long-term effectiveness of TPDN to a standard physical therapy approach of manual therapy and exercise for patients with SAPS.
Specific aims and hypothesesAim 1: Compare 6-week, 6- month and 1-year clinical outcomes in patients with SAPS receive standard PT treatment versus standard PT and TPDN versus standard PT and sham TPDN.
Hypothesis for Aim 1: Patients receiving standard PT and TPDN will demonstrate greater improvements in clinical outcomes compared with subjects in the standard PT only or standard PT plus sham TPDN. There will be no differences between the standard PT alone or standard PT plus sham TPDN groups.
Aim 2: Compare 1-year HCU findings in patients with SAPS that receive standard PT treatment versus standard PT and TPDN versus standard PT and sham TPDN.
Hypothesis for Aim 2: Patients in the standard PT plus TPDN group will utilize less healthcare compared to patients in the standard PT alone and the standard PT plus sham TPDN. There will be no difference in HCU between the standard PT alone and standard PT plus sham TPDN groups.
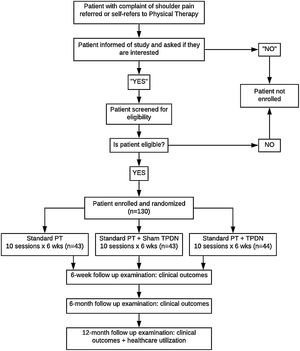
MethodsDesignThis will be a 3-arm, pragmatic comparative effectiveness randomized controlled trial. The study was designed following the standard protocol items for randomized interventional trials (SPIRIT) and the results will be reported in a manner consistent with the consolidated standards of reporting trials (CONSORT) guidelines.12 See Fig. 1 for subject flow through the study. Participants will be randomized to receive either standard PT interventions alone (consisting of manual therapy and exercise), standard PT and TPDN or standard PT and sham TPDN. The study has received approval from the Institutional Review Board at Wilford Hall Ambulatory Surgical Center (San Antonio, TX).
ParticipantsPatients will be recruited from two physical therapy clinics in the US Military Health System. Potential subjects with a primary complaint of unilateral shoulder pain may be referred to the PT clinics from other disciplines or self-refer to PT. Inclusion and exclusion criteria are described in Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Age 18–65 | 1. History of shoulder dislocation, subluxation, fracture, adhesive capsulitis of the glenohumeral joint, or cervical/shoulder/upper back surgery |
| 2. Read and speak English | 2. Presence of cervical radiculopathy, radiculitis, or referral from cervical spine |
| 3. Eligible for care in Military Health System | 3. Total baseline SPADI score less than 20% (to prevent a ceiling effect with treatment) |
| 4. Primary complaint of new episode of shoulder pain, defined as not having sought care for a shoulder condition in the preceding 6 months | 1. Pending a medical evaluation board, discharge from the military for medical reasons, or pending or undergoing litigation for an injury |
| 5. Willing to attend 8–12 treatment sessions over 6 week period | 2. Inability to give informed consent |
| 6. Meets physical exam criteria, as recommended by McClure and Michener13 and Diercks et al.3 Must have at least 2 of the following 4 findings: | 3. Pregnancy |
| a. Positive Impingement test (Hawkins-Kennedy or Neer tests) | |
| b. Painful arc | |
| c. Pain with isometric resistance (internal/external rotation, flexion, abduction) | |
| d. Rotator cuff weakness on the injured side in comparison to the opposite side |
Subjects will be randomized into one of three arms (Group I=Standard PT Treatment PLUS TPDN, Group II=Standard PT Treatment PLUS Sham TPDN, Group III=Standard PT Treatment only). The method of group assignment will be sequentially numbered opaque sealed envelopes in order to provide allocation concealment. To minimize the risk of predicting the treatment assignment of the next eligible patient, randomization will be performed in permuted blocks of 2 or 4 with random variation of the blocking number. All self-report measures will be filled out prior to randomization.
BlindingDue to the nature of this study, it is not possible to fully blind the patient or the clinician providing the intervention to the treatment received. The patient will be blinded to whether they receive real or sham TPDN but they will know if they are in the group that does not receive either. Outcome measures collected at baseline and all follow-up examinations will be filled out electronically by the subject and the treating clinicians will be blinded to these results. The incidence of unmasking will be recorded.
Treatment proceduresTreatment will begin immediately following subjects allocation to one of the three treatment groups. All subjects will attend 10 visits over the course of 6 weeks. The interventions delivered in each group are described below.
Standard PT treatment groupSubjects in the Standard PT Treatment (SPT) group will receive exercise and manual therapy interventions.
Exercise interventionsThe exercise program was adapted primarily from Tate et al.’s14 case series and informed from other published studies involving exercise in the treatment of SAPS.15 The program consists of six strengthening exercises and four stretching exercises (see Appendix 1). The six strengthening exercises may be progressed through three phases according to the patients evolving clinical presentation. Each exercise progression is designed to isolate specific rotator cuff or peri-scapular muscles in the initial phase and progress to exercises that require additional shoulder and trunk muscle activation, thus mimicking athletic movements and functional activities of daily living.16 Additionally, subjects will perform between one and three flexibility exercises, which include posterior shoulder stretching, anterior shoulder stretching and an exercise to improve shoulder elevation mobility. The same exercise program will be used for both the in-clinic and the home exercise program. The treating clinicians will spend 10–15min of each session instructing and supervising subjects performing the exercises and adjusting and progressing the home program. The details of the program are below.
Managing exercise associated pain: Exercises will be adjusted to minimize pain as much as possible with the goal of the exercise being pain-free. If an exercise is still painful despite attempts to modify it, a rating of 5/10 on the Numerical Pain Rating Scale (NPRS) will be set as the upper limit for acceptable pain during an exercise.15 If the exercise cannot be adjusted (i.e. reducing load or limiting the range of motion) to meet this pain threshold, it will be discontinued until the next supervised exercise session at which time it will be re-attempted. If soreness following an exercise session (either supervised or home exercise session) has not resolved to baseline levels by the following day, the exercise will be adjusted (e.g. the amount of resistance) to make it more tolerable.
Exercise progression: Subjects will begin with the lightest resistance (i.e. yellow tubing or 1–2lb weight), with the intent of allowing for pain-free movement and proper form. When subjects can perform an exercise for 3 sets of 15 repetitions without pain or difficulty, the resistance may be increased by selecting a stronger rubber tubing tension or increasing the amount of weight (i.e. dumbbell) used. When the clinician determines the subject has maximized the amount of resistance they can perform for a given phase of the exercise they will be progressed to the next phase.
Scapular positioning: Treating clinicians will provide instruction and ongoing feedback to improve subjects awareness and control of their scapula during shoulder exercises. For example, subjects will be cued to retract and depress the scapula prior to initiating glenohumeral movement and to monitor the scapula's position throughout the movement.
Exercise dosing and frequency: All six strengthening exercises will be prescribed and performed in 3 sets of 15 repetitions per session. Clinicians will have the choice of which stretching exercises to administer depending on the subjects observed impairments. One to three stretching exercises performed in 3 bouts of 60s will be administered. Subjects will be instructed to perform the prescribed home program once per day, every day for the first 12 weeks. After 12 weeks, the instruction will be to perform 3–4 days per week for an additional three months.
Manual therapy interventionsAll subjects will receive manual therapy (MT) interventions. Treating clinicians will examine the shoulder complex, cervical spine, thoracic spine and ribcage, and the surrounding musculature for mobility and flexibility deficits. Clinicians will select manual therapy techniques from a predetermined set of interventions to address impairments identified during the patient examination. Techniques will include non-thrust joint mobilization, thrust joint manipulation, and muscle stretching techniques. Similar to the protocol published by Tate et al. the manual therapy portion of the treatment session will last no longer than 15min and will include at least one technique targeting the posterior capsule, one thoracic spine technique and one technique to improve caudal glide of the humerus.14 Clinicians will be free to deliver additional techniques to these and other regions including the cervical spine, acromioclavicular joint, sternoclavicular joint, scapulothoracic joint and rib cage. All physical therapists delivering the interventions are familiar with the MT techniques used in the trial. Additionally, all clinicians underwent two 2-h training sessions with the one of the researchers (BH) to ensure competency in the MT techniques.
SPT plus TPDN groupIn addition to the SPT interventions, subjects in the SPT plus TPDN group will receive one TPDN session per week for a total of 6 TPDN sessions. The needling intervention will occur at the same visit as the SPT interventions. The determination of six sessions was made by discussion amongst members of the research team and input from physical therapists in the US military and civilian sector regarding their typical practice patterns.
TPDN interventionsThe dry needling portion of the treatment session will begin with an evaluation of the neck and shoulder musculature for the presence of latent and/or active myofascial trigger points. The following muscles will be evaluated: subscapularis, teres minor, supraspinatus, infraspinatus, deltoid (anterior, middle, posterior), trapezius (upper, middle, lower), levator scapulae, pectoralis major, latissimus dorsi, rhomboids (major, minor), biceps brachii and coracobrachialis. No more than three muscles will be treated per session. Therapists will use clinical reasoning to determine which muscles to treat at each session based on findings from the physical and historical examination.
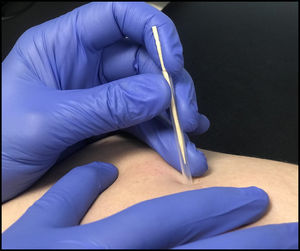
Once the muscles and specific myofascial trigger points to be treated have been localized, the therapists will use “clean technique” which is defined as hand-washing, the use of non-sterile gloves and an alcohol wipe to clean the skin prior to needling.17 Seirin J-type disposable, stainless steel needles (Seirin Corp., Shizuoka, Japan) will be used. The needle size will vary depending on the muscle targeted and will range from 0.30mm×40mm for thin muscles such as the infraspinatus to 0.30mm×60mm for larger muscles such as the latissimus or pectoralis major. The needle will be inserted directly into the myofascial trigger point at a depth of approximately 10–40mm appropriate to the thickness of the muscle (see Fig. 2.) After needle insertion, clinicians will perform a ‘sparrow pecking’ technique of moving the needle up and down approximately 3–5mm at 1Hz frequency in an attempt to elicit a local twitch response.18 If no local twitch responses are elicited, the needle may be redirected by withdrawing the needle toward the skin but not out of the skin, then redirecting the needle toward the suspected myofascial trigger point at a new angle.19 When a twitch response is observed, the pistoning will continue until the twitch response abates, there is decreased resistance to palpation of the tissue under question or patient intolerance to continued needling at this site develops.19 If no twitch response is produced, the treating clinician will attempt to elicit sensations of achiness, soreness, pressure or a reproduction of the patient's symptoms.20 Treatment of each myofascial trigger point will last from five to 30s depending on the patient and tissue response.19 Following needle removal, the treating clinician will perform gentle soft-tissue mobilization to the area treated for five to ten seconds. The number of myofascial trigger points treated in each muscle will be determined by the treating clinician and based on the number of identified trigger points, tissue response to the treatment, patient tolerance and post-treatment soreness considerations.20
SPT plus Sham TPDN groupSubjects in the standard PT intervention plus sham dry needling will receive 10 visits of SPT as described above plus 6 sessions of a sham TPDN procedure that will simulate the actual TPDN intervention. The sham needling intervention will occur at the same visit as the SPT interventions.
Sham TPDN Interventions: The sham procedure was adapted from the methods described by Sherman and colleagues.21 The evaluation for the presence and location of myofascial trigger points will be identical to the procedure described for the actual DN intervention. Subjects in the sham TPDN group will also receive six sessions (one per week) to no more than 3 muscles per session and clean technique will be used throughout the procedure.17 The clinician will don gloves, clean the target area with an alcohol wipe and place a guide tube with a toothpick firmly against the skin over the pre-identified myofascial trigger point. Care will be taken during this step to ensure the toothpick does not contact the skin (see Fig. 3). Next, the clinician will tap the other end of the toothpick to mimic the needle entering the skin. While the skin is held taut with one hand, the other hand will move the opposite end of the toothpick in a slow, circular motion while maintaining gentle downward pressure on the toothpick. This is intended to replicate the pistoning and changing angles of the needle penetration in the actual DN procedure. This will be performed for five to ten seconds. The toothpick will then be removed from the skin and the clinician will perform gentle soft-tissue mobilization to the area for five to ten seconds. This procedure will be repeated for each MrTP identified during the physical examination, using the same guiding principles as those used for the actual TPDN intervention. See Appendix 2 for step by step instructions of the sham TPDN procedures.
Subjects in the sham and actual DN treatment groups will be blinded to whether they are receiving actual or sham DN. If a muscle is being treated that is located on the anterior shoulder or chest wall, a towel will be placed over the subject's eyes so they cannot directly observe the treatment. For the posterior shoulder and peri-scapular muscles, the patient will be positioned prone and therefore unable to visualize the intervention.
Data collectionDependent variables (DV) will consist of patient-reported outcome (PRO) measures and health care utilization. PRO's will be collected at baseline, 6-weeks, 6-months, and 1-year post-enrollment. These measures will include the following:
Demographic informationPatients will self-report a variety of demographic and descriptive information including age, gender, ethnicity, job title, employment history, and current employment status as is standard of care for any medical appointment. Demographic information will be collected at the baseline examination only.
Shoulder Pain and Disability Index (SPADI)The SPADI is a 100-point, 13 item self-administered questionnaire that assesses pain and disability. Williams et al. have shown that the SPADI is responsive to change and accurately discriminates between patients who are improving or worsening.22 Michener and Leggin reported high test–retest reliability and internal consistency for this instrument.23 A recent systematic review identified a minimal detectable change (MDC) of 18 and a minimally clinically important difference (MCID) of between 8 and 13 points.24
Because disability will be used as an outcome of interest, it is important to ensure a moderate level of disability will be present at the inception of the treatment. Thus, patients will be required to have at least a baseline SPADI score of 20 points (average of pain and disability subscales). A score of 0 indicates no pain or functional limitation with described activities.
Patient Reported Outcomes Measurement Information Systems (PROMIS-57)The PROMIS 57-item short form (version 2.0) assesses several outcomes important to patients with shoulder disorders, including pain intensity and interference, sleep disturbance, anxiety, depression, fatigue, and social role participation.25 PROMIS-57 scores have been found valid for patients with shoulder disorders,26 with minimum clinically important change thresholds of 2–4 points for most scales.27
Patient Acceptable Symptom Scale (PASS)This outcome measure has been used in previous studies looking at the effects of cervical thoracic thrust manipulation on shoulder pain.28 The question that will be asked to assess this level is, “Taking into account all the activities you have during your daily life, your level of pain, and also your functional impairment, do you consider that your current state is satisfactory?” Individuals who responded “yes” were categorized as a success.”28 Between-group differences at three time points will be assessed as a percentage of subjects who answer “yes.”
Optimal Screening for Prediction of Referral and Outcome Yellow Flags assessment tool (OSPRO-YF)The OSPRO-YF is a screening tool used to identify pain-associated psychological distress, or yellow flags, in individuals with musculoskeletal pain.29 It allows for efficient and accurate assessment of factors that can affect an individual's potential for recovery, including depressive symptoms, anger, fear-avoidance beliefs, anxiety, kinesiophobia, catastrophizing, self-efficacy and pain acceptance.29 The OSPRO-YF was shown to have good concurrent validity with established measures of pain and disability in individuals with shoulder pain.29 We will use the 10-item version of the tool, in which scores range from 3 to 53.30 Higher scores indicate higher levels of psychological distress.30 OSPRO-YF scores will be used as secondary outcome measures and possible mediating factors.
Healthcare utilization (HCU)HCU data will be collected from the MHS Data Repository (MDR) database and will be confirmed through the electronic medical record used in the MHS. These data (type, location, number of clinic visits, types of specialty clinic visits, imaging, and associated medication) will be used to determine any subsequent medical utilization related to shoulder pain in each of the subjects. Groups will be analyzed for differences in HCU over the 12 months following study enrollment.
Clinician treatment logClinicians will record the details of the interventions delivered at each treatment session. The manual therapy techniques, exercises, muscles receiving TPDN and the patient education topics discussed will be logged.
Data analysisSample size and power calculationIn order to generate 80% power for this study, with 3 potential treatment arms, based an effect size difference in SPADI scores of 12%, we need a total sample size of 108 subjects (36 per group). With an estimated 20% loss to follow-up or dropouts at 1 year, we will plan on enrolling 130 subjects into the study in order to have 108 complete their 1-year follow-ups. Calculations derived with G*Power.31
Statistical approachAll data will be analyzed in IBM SPSS 24 (Chicago, IL). Descriptive statistics will be performed to describe the socio-demographic (age, sex, race, etc.) and health characteristics (disability, pain intensity, psychosocial factors, etc.) of the entire sample, and comparisons made between groups. Means and standard deviations will be computed for continuous data and frequency distributions will be analyzed for categorical data. For the primary endpoint (SPADI score at 1 year), a linear mixed effects model will be utilized to compare the differences in outcomes between the 3 groups at all time points. Linear mixed-effects models are ideal in this case as they allow for comparison of changes in the response variable over time, handle unbalanced data, and flexible in accounting for incomplete or missing data.32 If data is missing, a Little's Missing Completely at Random (MCAR) test will be run to validate the assumptions that data is missing at random and the appropriateness of the linear mixed-effects model.33 Subjects will be analyzed within the groups that they were initially randomized. For differences in shoulder-related healthcare utilization, RRs (risk ratio) for a healthcare utilization event between patients in each group will be derived and compared (e.g. injections, surgery, imaging). The significance is set at 0.05 and 95% confidence intervals will be reported for all relevant data. Sensitivity analysis will be run adjusting for other demographic and/or prognostic variables (OSPRO-YF, pain intensity, PROMIS sleep or mental health domains, clinical treatment log variables, etc.) that may affect the outcome and are different between groups at baseline.
ResultsData collection for this trial is currently underway and the trial is registered at ClinicalTrials.gov with the identifier: NCT03442894. Enrollment is expected to continue through 2019 with long-term outcomes completed by 2020 for all subjects.
DiscussionDespite a large number of interventional clinical trials, there is still considerable disagreement regarding the ideal treatment approach for individuals with SAPS.3 Effective non-surgical and non-pharmacological treatment strategies for SAPS investigated in the literature to date with the most supportive evidence include exercise therapy and manual therapy.34 Among available conservative treatment options, exercise therapy has the highest level of supportive evidence in the literature and is commonly recommended as the initial intervention for individuals with SAPS.35 Exercise has been shown to improve pain and shoulder function in individuals with SAPS.35 When compared with surgery, exercise has produced similar results and has also been shown to reduce the need for surgery in a proportion of patients.15 Several studies have shown added benefit from combining manual therapy and exercise over exercise alone.36
It is currently not known if TPDN provides long-term benefit for individuals with SAPS. We anticipate the results of this study will help determine if TPDN provides additive benefits over those observed with current commonly applied interventions of exercise and manual therapy.
Conflicts of interestThe authors declare no conflicts of interest.
Department of Defense Disclosure Statement: The views expressed are those of the author and do not reflect the official policy or position of the Department of Defense or the US Government.