Efficacy of cupping is known to be controversial, and the heterogeneity of the studies contributes to the uncertainty about its real benefits.
ObjectiveTo evaluate the effects of dry cupping therapy on pain, disability, functional capacity, and quality of life compared to sham cupping in women with knee osteoarthritis (KOA).
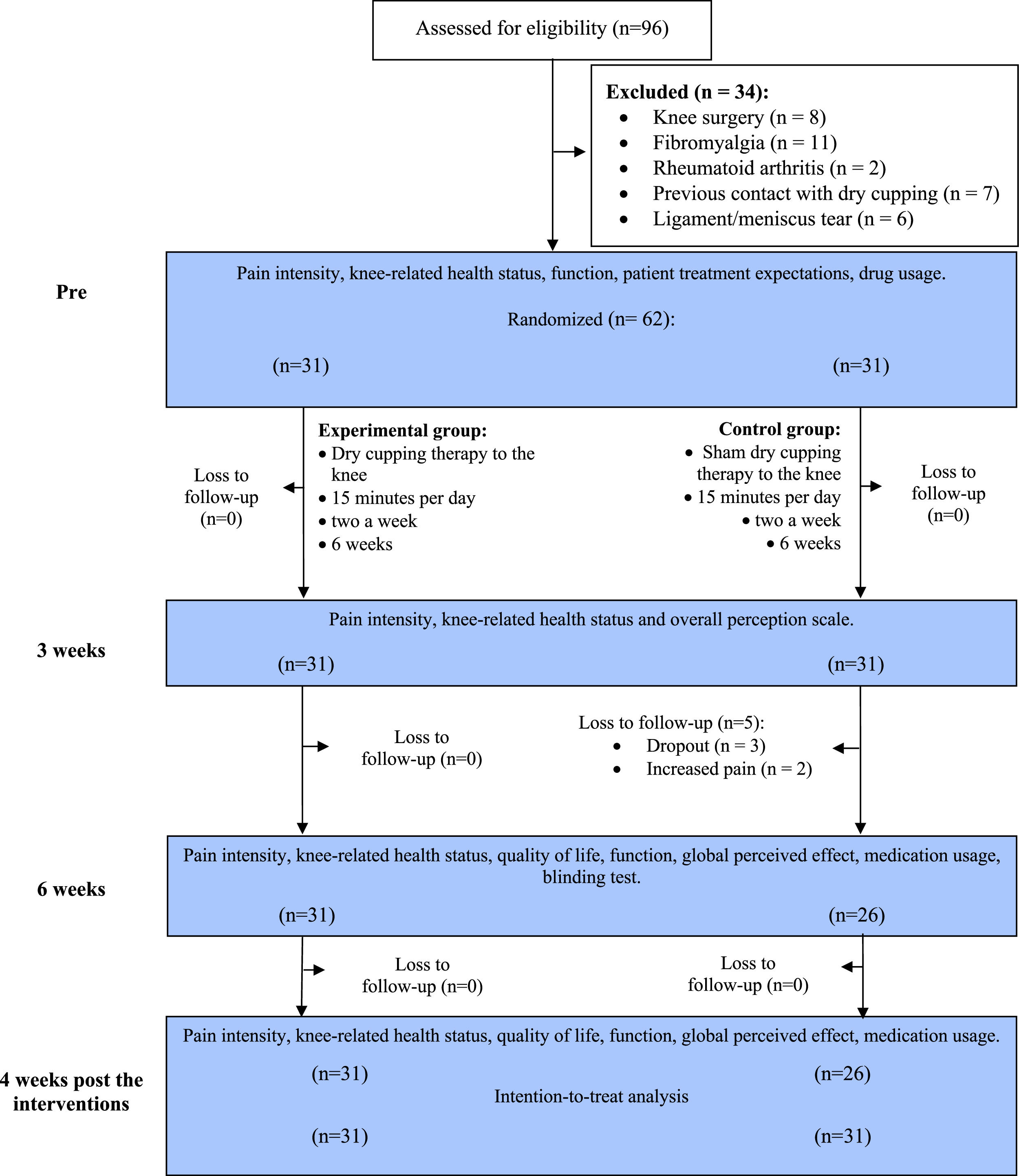
MethodsThis is a randomized placebo-controlled trial. Women with KOA aged 50–75 years were randomized into two groups: an experimental group, (receiving dry cupping therapy) and a control group (receiving sham cupping). Treatments consisted of 12 sessions, for 15 min, 2 × /week, for 6 consecutive weeks. Groups were evaluated at baseline (T0), 3 weeks after randomization (T3), at post-intervention or 6 weeks after randomization (T6), and 10 weeks after randomization (follow-up: T10). The primary outcome was pain intensity at rest or during movement, and secondary outcomes were the Global Perceived Effect (GPE), 30-seconds sit-to-stand test (STS-30), disability, 40-meter Fast-paced Walk Test (40mFPWT), and 8-step stair climb test (8-step SCT).
ResultsA total of 62 women with KOA were recruited. We observed a significant within-group reduction for the primary outcome (i.e., pain intensity at rest and during movement) and for some secondary outcomes, there were no differences between groups for all variables in any of the timepoints.
ConclusionDry cupping therapy was not superior to sham cupping in improving pain, functional capacity, and quality of life, and in GPE in women with knee osteoarthritis.
Trial registrationNCT04331158
Knee osteoarthritis (KOA) is more common in women and ranks among the leading causes of disability and chronic pain worldwide.1 It is characterized by cartilage degradation, subchondral bone changes, osteophytes, and local synovial inflammation, culminating in pain and disability.2 KOA also imposes a significant socioeconomic burden, due to its high treatment costs, and its associations with disability, early retirement from work, and even mortality.3–5
Clinical practice guidelines recognize that the therapeutic approach for people with KOA must include non-pharmacological strategies.6–8 Among these, cupping therapy has been widely used to treat pain9–11 and functional capacity.9 It is believed that suctioning the area may increase the supply of fresh blood to the area, thereby accelerating the removal of metabolites and promoting recovery. In addition, other theories have been proposed to explain the effects of dry cupping on pain, such as the pain gate theory, diffuse contractile inhibitory control, and reflex zone theory.12,13
Efficacy of cupping is known to be controversial, and the heterogeneity14 of the studies contributes to the uncertainty about its real benefits. Two systematic reviews12,15 showed high heterogeneity between studies and a high risk of bias. Several limitations were reported, such as lack of blinding, inadequate randomization, lack of follow-up, lack of a control group, and short intervention time,12,15 which weaken the certainty of the evidence and highlight the need for well-designed clinical trials with a robust methodology.12,15
The purpose of this study was to evaluate the effects of dry cupping on pain at rest and with movement in women with KOA. The secondary objectives were to evaluate the effects of dry cupping and sham cupping on functional performance, quality of life, use of analgesics and anti-inflammatories, and the degree of expectation and global perceived effect (GPE) with treatment after the intervention protocol. Our study was designed to address the limitations identified in previous research on the use of cupping in KOA, using a more robust protocol with 12 visits over 6 weeks, followed by a one-month post-intervention follow-up to assess both post-intervention and short-term effects.
MethodsStudy design and ethical aspectsThis is a randomized placebo-controlled trial with assessor and participant blinded, reported according to the Consolidated Standards of Reporting Trials (CONSORT) recommendations.16 The Osteoarthritis Research Society International (OARSI) consensus for designing, conducting, and reporting clinical trials in KOA17 and the Standards for Reporting Interventions in Clinical Trials of Cupping were also followed.18 All procedures were approved by the Research Ethics Committee of the Universidade Federal do Rio Grande do Norte (report number: 6.061.939), the trial was registered a priori (ClinicalTrials.gov identifier: NCT04331158), and the study protocol was previously described in detail.19 Study was conducted at the Physical Therapy Unit of the Hospital Universitário Onofre Lopes (Natal, RN, Brazil), between February and September 2023.
Sample size calculationSample size was calculated using G *Power software (version 3.1; University of Düsseldorf, Germany) ® with a statistical power of 80 % and an alpha of 5 %. Sample size was calculated based on the Numeric Pain Rating Scale (NPRS) from a previous study20 to detect a minimum between-group difference of 1.7 points, with a standard deviation of 2.25, in pain intensity as measured by the NPRS. Based on these calculations, a total of 62 participants were deemed necessary to ensure statistical power, given a 10 % dropout rate.
ParticipantsWe recruited women with KOA to participate in the study through social media, local TV and radio, and waiting lists at the hospital physical therapy unit. If both knees exhibited KOA, the limb with the highest level of pain was provided with intervention. Participants were randomized into two groups: an experimental group, that received dry cupping therapy, and a control group, that received sham cupping. We included participants aged 50−75 years21 with KOA according to the American College of Rheumatology,22 knee pain between 3 and 8 on the NPRS,23 and a body mass index < 35 kg/m2.
We excluded participants who: had been previously treated with cupping therapy on any part of the body,24 had contraindications to dry cupping therapy,12 were undergoing physical therapy for the knee, engaged in physical activity (> 45 min/week),25 were diagnosed with fibromyalgia,26 had clinically diagnosed musculoskeletal dysfunctions, had meniscal injuries (positive Apley test),27 ankle, knee, or hip surgery,27 systemic diseases,27 neurological, vestibular, visual, or auditory deficits, or any medical restriction that would prevent them from participating in the proposed assessments and interventions.25 Participants were excluded if they were undergoing or had undergone physical therapy for their knee in the 3 months prior to the survey, were considering the use of corticosteroid injections in the knee in the last 6 months, and were planning to travel in the following 2 months after the start of the study.
Randomization and strategies to decrease the risk of biasThe study involved four researchers who were responsible for the randomization of participants, assessments, interventions, and data analysis. Participants were screened for eligibility by a physical therapist. A blinded assessor conducted assessments of outcome measures for all participants at baseline (T0), at three weeks after randomization (T3) and post-intervention at six weeks (T6) and 10 weeks after randomization (T10).
Randomization was conducted by an independent researcher. Participants were assigned a number from the randomization table (generated by the website randomization.com) for enrollment, corresponding to the experimental or control group. The allocation concealment was ensured using sealed, opaque, sequentially numbered envelopes. The group allocation of each participant was only revealed to the physical therapist who administered the treatments immediately before the first intervention. Participants were informed that they would receive two different dry cupping therapies: one real and one sham.
During all evaluations, participants performed the functional tests wearing clothing that covered their knees and were instructed to remain in this type of clothing after each service to cover the treated area, taking into account the possibility of bruising resulting from the application of dry cupping therapy. This measure was taken to ensure that the other participants and the blind evaluator could not identify which group each participant belonged to. In addition, intervention times were scheduled to avoid contact between participants of different groups. Participants were also instructed not to comment to the blind evaluator or anyone else during the reassessments about the procedure they believed they were undergoing during the interventions.
Researcher responsible for the statistical analysis remained blind. At the end of the intervention, they received a data table with all the necessary information, without identification of the participants and groups. The names of the groups were replaced in the data table, and only at the end of the analyses, the groups were revealed to the statistician.
Outcome measuresA detailed description of the outcome measures was presented in the previously published study protocol.19 A detailed description of the assessment instruments for each outcome is presented in Supplementary Material 1.
In addition to these main outcome measures, participants’ treatment expectations were also measured at T0 using a 5-point Likert scale. Participants indicated a score of 1 if they believed the treatment would make them “worse” and 5 if they believed the treatment would make them “much better”.28 Studies suggest that expectations may influence pain outcomes in randomized control trials.11 Medication use was recorded before each intervention.
The protocol 19 was published prior to the initiation of the recruitment process. In these, we formally announced the incorporation of secondary outcomes that were not documented during the clinical trial. Following the finalization of the study report, the secondary outcomes that had been prospectively declared in the protocol were incorporated.19
At the end of the interventions, participants were asked about the suction sensation they felt during the treatments regarding the location and intensity of the sensation (0 = no sensation, 100 = very strong sensation).29 Finally, in the post-intervention assessment, participants were asked which treatment they thought they had received to verify the effectiveness of the blinding strategy.
InterventionsTreatments consisted of 12 sessions of dry cupping therapy or sham cupping, performed for 15 min, twice a week, for 6 consecutive weeks.19 Participants were placed in a supine position on a couch with a wedge to keep the torso reclined for better comfort. A roller was placed on the posterior region of the knee to maintain it at 15° of flexion (0° = full knee extension).19 Finally, an opaque structure was placed transversely on the participant’s thigh to ensure that the participant could not see their knees during the intervention.
All participants were informed that they might feel a suction sensation and bruising at the dry cupping application sites. The application site of dry cupping therapy was around the knee at the apex of the patella, on the medial and lateral edge of the patella, medial and lateral to the patellar tendon,19 just like its acupuncture equivalents, according to previous studies.30–32 Two different sizes of suction cups were used to cover most of the area and provide better fixation according to the anatomy of the knee.19 A manual suction pump and five Dong Yang® acrylic suction cups were used for each group, three size one (internal diameter = 4.5 cm) and two size two (internal diameter = 2.3 cm).19
For the experimental group, dry cupping therapy was applied with the force of two suctions of the pump, creating a slight negative pressure (between 100 and less than 300 millibars) on the skin.19 In the control group, the cups were prepared with small holes (< 2 mm in diameter) to release the negative pressure in approximately 3 s. Transparent double-sided adhesive tape was applied to the rim of the cups to keep them in contact with the participants’ skin.19,24
Statistical analysisAll participants were kept in their respective groups, according to the intention-to-treat principle, as such, the multiple imputation method was used to fill in missing data. We used alpha < 0.05 for all statistical analyses, which were performed using the commercial statistical software SPSS 20.0® (SPSS Inc, Chicago, USA). An investigator who was not involved in the recruitment, assessment, or intervention aspects of the study performed all statistical analyses.33,34
Interaction effects between groups × time (T0, T3, T6, and T10) were evaluated using generalized estimating equations analysis.35 In all models, the outcome variables were analyzed as dependent variables, while the groups and times were analyzed as independent variables with repeated measures.35 To determine the best likelihood function and the best correlation matrix of the models, the quasi-likelihood was used under the independence model criterion, where a lower value indicates a better model fit.36 Thus, the normal probability, the identity link function, and the independent correlation matrix were adopted. The confidence intervals (95 %CI) for the comparisons were obtained using the Bonferroni test.
ResultsA total of 96 participants were initially selected for the study. Of these, 62 met the eligibility criteria and were randomized after the initial assessment. Of the randomized participants, 57 completed all intervention procedures. Five participants in the control group discontinued treatment after the third week, resulting in an 8 % dropout rate. The multiple imputation method outlined in the statistical analysis was used for the missing data. Reasons for dropping out included increased knee pain after cup application. No participants in the experimental group discontinued treatment (Fig. 1).
During the intervention period, 27 participants (87 %) in the experimental group used analgesics and/or anti-inflammatories, while 26 participants (84 %) in the control group used these medications. In terms of the number of absences during treatment sessions, there were no more than three absences per participant in either group.
Demographic characteristics of participants in both groups were similar at T0. The mean (standard deviation [SD]) age and mean body mass index were 60.4 (6.3) years and 30.8 (4.7) kg/m2 for the experimental group and 60.0 (5.7) years and 31.7 (4.5) kg/m2 for the control group. Approximately 55 % of the sample reported the right knee as the most troublesome knee, and more than 85 % were taking medication at the time of evaluation. Other sample characteristics are described in Table 1.
Characteristics of the participants. Mean (standard deviation) or n ( %).
| Variable | Experimental group (n = 31) | Control group (n = 31) |
|---|---|---|
| Age (years) | 60.4 (6.3) | 60.0 (5.7) |
| Body mass index (kg/m2) | 30.8 (4.7) | 31.7 (4.5) |
| Ethnicity, n ( %)* | ||
| White | 11 (35.5) | 9 (20.9) |
| Yellow | 1 (3.2) | 0 (0) |
| Brown | 11 (35.5) | 19 (61.3) |
| Black | 8 (25.8) | 3 (9.7) |
| Education | ||
| Incomplete primary education | 12 (38.7) | 11 (35.5) |
| Complete primary education | 0 (0.0) | 2 (6.5) |
| Incomplete high school | 2 (6.5) | 4 (12.9) |
| Complete high school | 11 (35.5) | 5 (16.1) |
| Complete graduate | 4 (12.9) | 5 (16.1) |
| Complete postgraduate | 2 (6.5) | 2 (6.5) |
| Illiterate | 0 | 2 (6.5) |
| Monthly earned (R$ per month) | ||
| 1412.00 | 13 (41.9) | 15 (48.4) |
| 1412.00 – 2824.00 | 11 (35.5) | 10 (32.3) |
| 2824.00 – 4236.00 | 7 (22.6) | 3 (9.7) |
| 4236.00 – 7060.00 | 0 (0.0) | 2 (6.5) |
| > 7060.00 | 0 (0.0) | 1 (3.2) |
| Most troublesome knee | ||
| Left | 14 (45.2) | 14 (45.2) |
| Right | 17 (54.8) | 17 (54.8) |
| Medication (yes) | 26 (83.9) | 27 (87.1) |
| Patient treatment expectations (1–5), | 4.8 (0.4) | 4.7 (0.5) |
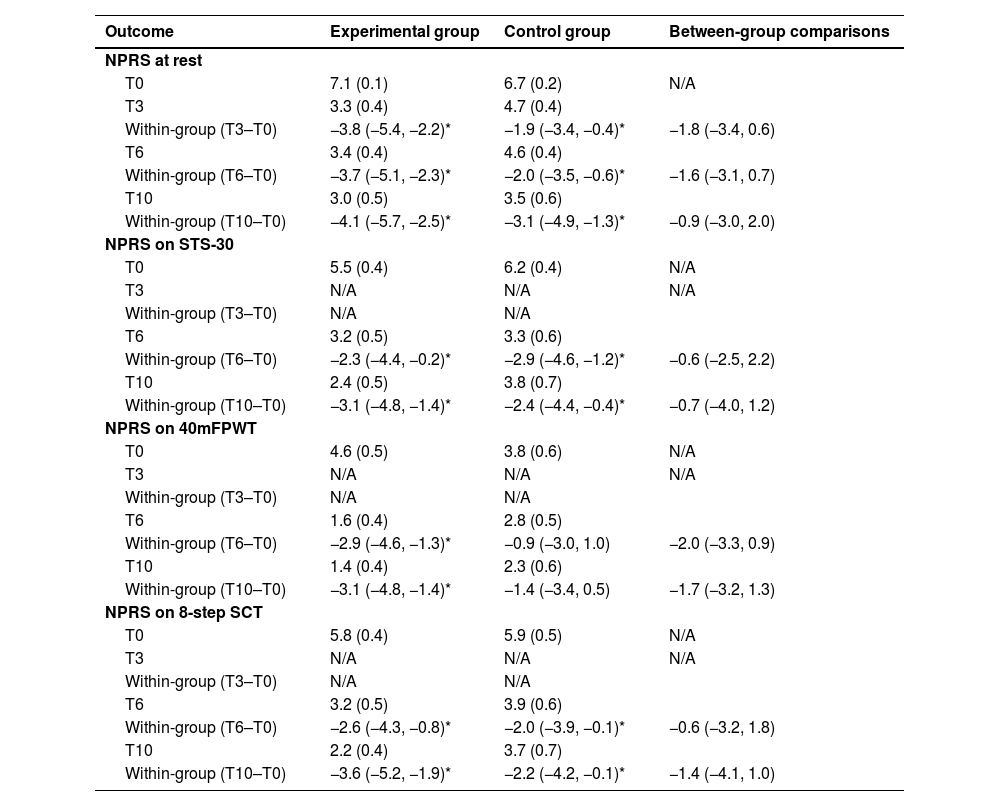
Tables 2 and 3 present the clinical data for both groups at T0, T3, T6, and T10 (follow-up). The between-group and within-group differences are presented for all outcomes, including pain at rest and during movement, functional capacity tests (i.e., STS-30, 8-step SCT, 40mFPWT), WOMAC, quality of life, and GPE at different time points. No between-group differences for the primary outcomes, pain at rest and during movement, and any of the secondary outcomes were observed. Only within-group differences for some variables were observed.
Mean (SD) of the groups and difference of the means (95 %CI): primary outcome.
| Outcome | Experimental group | Control group | Between-group comparisons |
|---|---|---|---|
| NPRS at rest | |||
| T0 | 7.1 (0.1) | 6.7 (0.2) | N/A |
| T3 | 3.3 (0.4) | 4.7 (0.4) | |
| Within-group (T3–T0) | −3.8 (−5.4, −2.2)* | −1.9 (−3.4, −0.4)* | −1.8 (−3.4, 0.6) |
| T6 | 3.4 (0.4) | 4.6 (0.4) | |
| Within-group (T6–T0) | −3.7 (−5.1, −2.3)* | −2.0 (−3.5, −0.6)* | −1.6 (−3.1, 0.7) |
| T10 | 3.0 (0.5) | 3.5 (0.6) | |
| Within-group (T10–T0) | −4.1 (−5.7, −2.5)* | −3.1 (−4.9, −1.3)* | −0.9 (−3.0, 2.0) |
| NPRS on STS-30 | |||
| T0 | 5.5 (0.4) | 6.2 (0.4) | N/A |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | |
| T6 | 3.2 (0.5) | 3.3 (0.6) | |
| Within-group (T6–T0) | −2.3 (−4.4, −0.2)* | −2.9 (−4.6, −1.2)* | −0.6 (−2.5, 2.2) |
| T10 | 2.4 (0.5) | 3.8 (0.7) | |
| Within-group (T10–T0) | −3.1 (−4.8, −1.4)* | −2.4 (−4.4, −0.4)* | −0.7 (−4.0, 1.2) |
| NPRS on 40mFPWT | |||
| T0 | 4.6 (0.5) | 3.8 (0.6) | N/A |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | |
| T6 | 1.6 (0.4) | 2.8 (0.5) | |
| Within-group (T6–T0) | −2.9 (−4.6, −1.3)* | −0.9 (−3.0, 1.0) | −2.0 (−3.3, 0.9) |
| T10 | 1.4 (0.4) | 2.3 (0.6) | |
| Within-group (T10–T0) | −3.1 (−4.8, −1.4)* | −1.4 (−3.4, 0.5) | −1.7 (−3.2, 1.3) |
| NPRS on 8-step SCT | |||
| T0 | 5.8 (0.4) | 5.9 (0.5) | N/A |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | |
| T6 | 3.2 (0.5) | 3.9 (0.6) | |
| Within-group (T6–T0) | −2.6 (−4.3, −0.8)* | −2.0 (−3.9, −0.1)* | −0.6 (−3.2, 1.8) |
| T10 | 2.2 (0.4) | 3.7 (0.7) | |
| Within-group (T10–T0) | −3.6 (−5.2, −1.9)* | −2.2 (−4.2, −0.1)* | −1.4 (−4.1, 1.0) |
95 %CI, 95 % confidence interval; 40mFPWT, 40-meter Fast-paced Walk Test; 8-step SCT, 8-step stair climb test; N/A. Not applicable; NPRS, Numeric Pain Rating Scale; SD, standard deviation; STS-30, 30-seconds sit-to-stand test; T0, baseline; T3, 3 weeks; T6, 6 weeks; T10, 10 weeks.
Mean (SD) of the groups and difference of the means (95 %CI): secondary outcome.
| Outcome | Experimental group | Control group | Between-group comparisons |
|---|---|---|---|
| STS-30 (rep) | |||
| T0 | 7.3 (0.4) | 8.0 (0.3) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 9.0 (0.4) | 8.8 (0.4) | |
| Within-group (T6–T0) | 1.6 (0.3, 2.9)* | 0.7 (−0.2, 1.7) | 0.9 (−1.6; 2.1) |
| T10 | 9.7 (0.4) | 8.7 (0.4) | |
| Within-group (T10–T0) | 2.3 (1.1, 3.4)* | 0.7 (−0.5, 1.9) | 1.7 (−0.8, 2.8) |
| 40mFPWT (time) | |||
| T0 | 44.6 (1.8) | 41.3 (1.8) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 40.9 (1.4) | 40.5 (1.6) | |
| Within-group (T6–T0) | −3.7 (−7.9, 0.4) | −0.8 (−8.1, 6.4) | 2.9 (−6.2, 6.9) |
| T10 | 40.3 (1.6) | 41.8 (2.4) | |
| Within-group (T10–T0) | −4.3 (−8.5, −0.1)* | 0.5 (−10.1, 11.1) | −4.8 (−10.2, 7.1) |
| 8-step SCT (time) | |||
| T0 | 21.3 (1.5) | 22.5 (2.7) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 18.3 (1.5) | 19.6 (1.4) | |
| Within-group (T6–T0) | −2.9 (−5.7, −0.1) | −2.9 (−10.6, 4.8) | −0.1 (−7.4, 4.9) |
| T10 | 17.1 (1.5) | 20.6 (2.9) | |
| Within-group (T10–T0) | −4.2 (−7.1, −1.3) | −1.9 (−12.8, 8.9) | −2.3 (−13.3, 6.2) |
| WOMAC (0–96) | |||
| T0 | 40.5 (2.5) | 39.6 (2.4) | |
| T3 | 24.2 (2.5) | 31.2 (2.7) | N/A |
| Within-group (T3–T0) | −16.2 (−25.8, −6.6)* | −8.3 (−16.7, 0.0)* | −7.9 (−18.5, 4.5) |
| T6 | 19.9 (2.4) | 23.6 (3.4) | |
| Within-group (T6–T0) | −20.5 (−31.6, −9.4)* | −16.0 (−24.5, −7.4)* | −4.6 (−16.7, 9.4) |
| T10 | 17.8 (2.8) | 23.3 (3.6) | |
| Within-group (T10–T0) | −22.6 (−33.4, −1.8)* | −16.2 (−25.2, −7.2)* | −6.4 (−19.8, 8.7) |
| SF-36 (0–100) | |||
| Functional capacity | |||
| T0 | 36.9 (4.2) | 38.7 (4.1) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 55.9 (4.1) | 52.1 (4.8) | |
| Within-group (T6–T0) | 19.0 (6.4, 31.6)* | 13.4 (0.6, 26.2)* | 5.6 (−14.8, 22.5) |
| T10 | 58.3 (4.2) | 52.5 (4.8) | |
| Within-group (T10–T0) | 19.6 (2.2, 37.1)* | 13.7 (0.6, 26.9)* | 7.6 (−13.1, 24.9) |
| Physical limitation | |||
| T0 | 20.1 (6.0) | 17.7 (6.2) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 34.6 (7.8) | 36.5 (7.9) | |
| Within-group (T6–T0) | 14.5 (−9.5, 38.6) | 18.8 (−7.7, 45.3) | −4.3 (−34.7, 31.0) |
| T10 | 42.7 (8.1) | 43.2 (7.8) | |
| Within-group (T10–T0) | 22.5 (−2.3, 47.4) | 25.5 (−0.7, 51.7) | −2.9 (−33.8, 32.7) |
| Pain | |||
| T0 | 36.9 (2.9) | 44.6 (3.5) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 50.4 (4.0) | 48.9 (3.0) | |
| Within-group (T6–T0) | 13.5 (4.0, 22.9)* | 4.3 (−0.4, 24.3) | 9.2 (−13.2, 16.3) |
| T10 | 47.7 (3.6) | 50.7 (3.8) | |
| Within-group (T10–T0) | 10.8 (3.1, 18.4)* | 6.1 (−0.3, 27.8) | −4.7 (−18.5, 12.5) |
| General health status | |||
| T0 | 57.7 (4.0) | 55.4 (4.3) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 61.1 (3.9) | 56.1 (4.7) | |
| Within-group (T6–T0) | 3.4 (−4.9, 11.8) | 0.7 (−10.8, 12.2) | 2.7 (−13.0, 23.1) |
| T10 | 63.7 (4.2) | 55.0 (5.0) | |
| Within-group (T10–T0) | 6.0 (−2.4, 14.5) | −0.3 (−13.1, 12.4) | 6.4 (−10.7, 28.0) |
| Vitality | |||
| T0 | 52.1 (3.7) | 48.0 (4.6) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 59.5 (4.2) | 54.6 (4.4) | |
| Within-group (T6–T0) | 7.4 (−4.0, 18.9) | 6.5 (−8.7, 21.8) | 0.8 (−13.1, 22.9) |
| T10 | 55.0 (4.2) | 57.6 (4.3) | |
| Within-group (T10–T0) | 2.9 (−8.5, 14.4) | 9.6 (−2.2, 21.5) | −6.7 (−20.5, 15.2) |
| Social aspects | |||
| T0 | 63.7 (5.6) | 58.0 (5.3) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 74.1 (4.9) | 74.5 (4.8) | |
| Within-group (T6–T0) | 10.4 (−4.0, 24.9) | 16.4 (−0.4, 33.3) | −6.1 (−20.5, 19.8) |
| T10 | 72.1 (5.5) | 73.0 (4.8) | |
| Within-group (T10–T0) | 8.4 (−5.0, 22.0) | 15.0 (0.7, 29.3)* | −6.6 (−22.6, 20.8) |
| Emotional aspects | |||
| T0 | 49.4 (8.3) | 31.1 (7.2) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 66.6 (7.7) | 42.3 (8.9) | |
| Within-group (T6–T0) | 17.2 (−7.6, 42.0) | 11.1 (−12.6, 34.8) | 6.0 (−10.4, 59.1) |
| T10 | 62.3 (8.2) | 51.2 (9.1) | |
| Within-group (T10–T0) | 12.9 (−12.2, 38.0) | 20.1 (−5.0, 45.2) | 7.2 (−25.0, 47.2) |
| Mental health | |||
| T0 | 66.1 (4.1) | 62.3 (4.1) | |
| T3 | N/A | N/A | N/A |
| Within-group (T3–T0) | N/A | N/A | N/A |
| T6 | 73.6 (3.5) | 61.8 (3.6) | |
| Within-group (T6–T0) | 7.4 (0.5, 14.4)* | −0.4 (−9.1, 8.1) | 8.0 (−3.1, 26.7) |
| T10 | 70.4 (4.1) | 66.3 (4.4) | |
| Within-group (T10–T0) | 4.2 (−2.6, 11.1) | 3.9 (−5.7, 13.7) | 0.3 (−13.6, 21.9) |
| GPE (+ 5, –5) | |||
| T0 | −0.5 (0.5) | −1.1 (0.4) | |
| T3 | 2.4 (0.2) | 1.3 (0.3) | N/A |
| Within-group (T3–T0) | 1.9 (1.4, 4.7)* | 0.2 (0.1, 4.1)* | 1.7 (−0.3, 2.4) |
| T6 | 3.1 (0.3) | 2.2 (0.3) | |
| Within-group (T6–T0) | 2.6 (1.9, 5.5)* | 1.1 (1.8, 5.0)* | 1.5 (−0.5, 2.4) |
| T10 | 2.5 (0.4) | 1.5 (0.4) | |
| Within-group (T10–T0) | 2.0 (1.0, 5.1)* | 0.4 (0.2, 4.5)* | 1.6 (−1.0, 2.9) |
95 %CI, 95 % Confidence interval; 40mFPWT, 40-meter Fast-paced Walk Test; 8-step SCT, 8-step stair climb test; N/A, Not applicable; NPRS, Numeric Pain Rating Scale; SD, Standard deviation; SF-36, Short Form-36; STS-30, 30-seconds sit-to-stand test; T0, baseline; T3, 3 weeks; T6, 6 weeks; T10, 10 weeks; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Regarding the effectiveness of the strategy to blind the participants, in the experimental group, 25 participants (81 %) believed they had received dry cupping therapy, while 6 participants (19 %) believed they had received sham therapy. In the control group, 19 participants (73 %) believed they had received dry cupping therapy, while 7 participants (27 %) believed they had received sham therapy. There was no difference between groups (p > 0.05) (Table 4).
Sensations experienced during dry cupping and sham cupping procedures, location of sensation, and intensity of sensation.
| Variable | Experimental (n = 31) | Control (n = 31) | p | w |
|---|---|---|---|---|
| What group do you think you were in? (Blinding) | ||||
| Dry cupping therapy | 25 (80.6) | 19 (73.1) | 0.49 | 0.18 |
| Sham cupping | 6 (19.4) | 7 (26.9) | ||
| Suction sensation | 31 (100) | 26 (92.3) | 0.20 | 0.42# |
| Sensations experienced | ||||
| Pressure | 28 (90.3) | 16 (61.5) | 0.01* | 0.72# |
| Inflation | 1 (3.2) | 3 (11.5) | 0.22 | 0.32# |
| Pain | 4 (12.9) | 5 (19.2) | 0.51 | 0.17 |
| Tighten | 27 (87.1) | 18 (69.2) | 0.09 | 0.44# |
| Relaxation | 20 (64.5) | 15 (57.7) | 0.59 | 0.14 |
| Refreshing | 6 (19.4) | 10 (38.5) | 0.11 | 0.43# |
| Burning | 6 (19.4) | 6 (23.1) | 0.73 | 0.09 |
| Pulling | 22 (71.0) | 16 (61.5) | 0.45 | 0.20 |
| Tingling | 11 (35.5) | 9 (34.6) | 0.94 | 0.01 |
| Sensation location | ||||
| Suction cup area | 16 (51.6) | 15 (57.7) | 0.72 | 0.21 |
| Around the area of the suction cup | 6 (19.4) | 3 (11.5) | ||
| Whole knee area | 9 (29.0) | 8 (30.8) | ||
| Sensation intensity (0–100) | 63.2 (23.9) | 61.9 (24.6) | 0.84 | 0.13 |
In the experimental group, 31 participants (100 %) reported that they felt a suction sensation during the interventions, with 16 of them (52 %) mentioning that they felt the sensation at the place where the treatment was applied (suction cup area). Mean (SD) intensity of the suction sensation reported by this group was 63.2 (24.0). In the control group, 24 participants (92.3 %) also reported feeling a suction sensation during the interventions, with 15 participants (57.7 %) mentioning that they felt the sensation at the place where the treatment was applied (suction cup area). The mean (SD) suction sensation reported by this group was 61.9 (24.6) (Table 4). In addition, when analyzing the participants’ reports of the characteristics of the sensations experienced, we observed that only the sensation of “pressure” showed a significant difference (p < 0.01). No adverse events were observed in the groups studied or reported by the participants.
DiscussionThis is the first clinical trial to compare dry cupping therapy with sham cupping in women with KOA, evaluating outcomes of pain intensity, functional capacity, quality of life, and perceived overall effect. At weeks three, six, and ten after initiating treatment, both groups showed similar reductions in pain intensity, with no significant differences between them at any time point. No clinically valid effects of dry cupping therapy were confirmed in secondary outcomes. These results question the efficacy of dry cupping therapy as a non-pharmacological option for the treatment of KOA.
Although a systematic review14 indicated that dry cupping therapy may reduce pain and improve functional capacity in people with KOA, the strength of this conclusion is undermined by the heterogeneity and low methodological quality of the studies analyzed. Therefore, these results must be interpreted with caution, as not all included studies had a control group for comparison, limiting the ability to evaluate the true effectiveness of dry cupping therapy. However, our findings, using a more robust methodological design with rigorous control, intention-to-treat analysis, a sham-controlled group, and appropriate comparison group, provide new evidence that may contribute to a more accurate assessment of the efficacy of this therapeutic approach for KOA.
Our results contrast with those of Teut et al., who reported significant improvements in pain, WOMAC, and quality of life after eight cupping sessions over four weeks.37 However, their study had methodological limitations such as the lack of a control group, lack of blinding, and did not perform an intention-to-treat analysis. Moreover, authors did not perform a sample size calculation, which raises concerns about the reliability of their findings. A small sample size increases the risk of type I error, which means that observed benefits may have been due to chance rather than a true effect of the intervention.37 Islam et al. showed a significant effect of cupping combined with deep massage in people with KOA, for improving outcomes such as pain, activities of daily living, recreational activities, and quality of life.38 The protocol consisted of 15-minute sessions on alternate days for a total of 10 sessions over 20 days.38 Nevertheless, this study also had a high risk of bias due to lack of blinding, small sample size, short duration, and lack of post-intervention follow-up (to assess medium-term efficacy), again raising the possibility of overestimating the effects of the intervention. In contrast, our findings, with a more robust methodological design, larger sample size, rigorous bias control, and adequate post-intervention follow-up, provide more reliable evidence and a more accurate assessment of the efficacy of this intervention in people with KOA.
Results of the present study also challenge the primary mechanisms proposed to explain the pain-relieving effects of dry cupping therapy for people with KOA. Three theoretical bases have been suggested to explain the physiological effects of pain reduction after the use of dry cupping therapy: the pain portal theory,39 the diffuse inhibitory control theory,40 and the reflex zone theory.12 However, these theories are unlikely to explain the totality of the analgesic effect, because the participants in the sham cupping group showed similar improvements to the dry cupping therapy group. Given the results of the current study, the placebo effect,41 positive expectations regarding the treatment,42 the therapist-patient alliance (Hawthorne effect),43 and the environmental context are factors that most likely explain the improvements observed in the participants44 with the cupping intervention.
For the secondary outcomes, the mean differences between groups clearly excluded the possibility of clinically significant effects of cupping on the functional capacity, quality of life, and global perceived effect. The estimated effects for secondary outcomes were below the minimum clinically significant thresholds, and all 95 % CIs indicated no effect. Therefore, there was no clinically significant effect of dry cupping therapy on any of the secondary outcomes for people with KOA.
Similarly, Islam et al. used the same intervention for people with KOA. However, their sample was small (total, n = 40) and weak (β = 90 %), whereas our sample size (n = 62) and statistical power were greater (β = 80 %); their a priori estimate of sample loss was 20 %, whereas ours was 10 %; they did not use an intention-to-treat in their data analysis, and their sample attrition exceeded 15 %, as suggested by the PEDro scale, whereas ours had 5 % dropout rate.38 The clinical implications of our findings are relevant to health care systems and clinicians. Given the lack of proven benefit of dry cupping therapy beyond placebo effects and other contextual factors, resources could be better allocated to interventions with stronger evidence of effectiveness, such as structured exercise programs and patient education. These findings highlight the importance of high-quality research to guide clinical decision making and prevent the proliferation of ineffective treatments.
Clinicians should be aware of the limited effectiveness of dry cupping therapy and consider prioritizing interventions such as exercise therapy and patient education, which have stronger scientific support, for the management of KOA.45,46 Additionally, educating patients about the evidence (or lack thereof) behind different treatment options is essential to promote informed decision-making and prevent the use of ineffective therapies that may lead to unrealistic expectations or unnecessary healthcare costs.47,48
There are limitations to our study that need to be considered. Only women were included; therefore, it is unknown whether these results are generalizable to men with KOA. Due to the nature of the intervention, it was not possible to blind the therapist responsible for delivering the intervention to the participants in both groups. The irregularity of the knee area, which varied according to the anatomy of each participant, made it difficult to apply the suction cups. In addition, more than 80 % of both groups were taking medications, which may be a confounding variable in terms of prognosis.49,50 Finally, the negative pressure created by the suction cups could vary from 100 to 300 millibars, and we did not evaluate the mean of these data; the conclusions of this study were limited to the application of dry cupping therapy in isolation, which may not reflect the treatment typically provided by therapists in their clinical practice. Future studies should consider including both sexes, standardizing suction pressure, and evaluating potential long-term effects beyond the intervention period.
ConclusionDry cupping therapy was not superior to sham cupping in improving pain, functional capacity, quality of life, and global perceived effect in women with KOA. Until more robust evidence of efficacy is available, practitioners should reconsider the use of dry cupping in people with KOA and advise them to consider interventions based on the best available evidence.
Authors’ contribution statementRRC and MCS – Conceptualization, Investigation, Data curation. All authors – Formal Analysis, Methodology, Validation, Visualization, Writing (original draft, review, and editing).
Availability of data and materialsThe data and materials in this paper are available from the corresponding author on request.
The authors declare no competing interests.
This study was partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, code 001). The funding source had no role in the study design, collection, analysis, interpretation of data, writing of the report, or in the decision to submit the article for publication. We extend our gratitude to all the patients who so kindly volunteered to participate in this research, thereby forming the study sample.