Transcutaneous electrical nerve stimulation (TENS) is a treatment commonly used for managing pain; however, the ideal placement of the electrodes is not fully understood.
ObjectiveTo investigate the best way to apply TENS electrodes in an experimental inflammatory pain model.
MethodKnee joint inflammation was induced in rats, followed by administration of low-frequency TENS (4Hz) under anesthesia for five days. Animals were randomly allocated to five groups according to electrode placement (n=6, each): dermatome, contralateral, paraspinal, acupoint, and control. Interventions: Low-frequency TENS at sensory intensity and 100μs pulse duration. Withdrawal thresholds to mechanical (von Frey) and thermal stimuli and joint edema were assessed before induction of inflammation and immediately before and after application of TENS.
ResultsReduced paw withdrawal threshold and thermal latency that occur 24h after the induction of inflammation were significantly reversed by the administration of TENS in all groups when compared with sham treatment or with the condition before TENS treatment. No difference was observed in the edema measurement.
ConclusionThese results offer more options for practitioners to choose the area of the body most commodious for electrode placement, depending on the clinical condition of the patient, because the effect was similar at all sites. In addition, there was a loss of the effectiveness of TENS in reversing mechanical and thermal hyperalgesia on the fifth day, suggesting the development of the tolerance phenomenon.
The American Physical Therapy Association defines Transcutaneous Electric Nerve Stimulation (TENS) as the application of electrical stimulation to the skin to control pain. TENS is a clinical treatment that is non-invasive, inexpensive, and easy to use. It is also safe and has few adverse side effects.1 These side effects were seen only in the burst TENS, which inhibited collagen I and III production and impaired its alignment during partial rupture of the Achilles tendon in rats.2
TENS stimulates large diameter peripheral nerves through electrodes placed on the skin to achieve therapeutic effects.3 The overall goal of TENS therapy is to relieve pain, thus allowing increased activity with less discomfort.4,5 However, despite the large number of clinical trials showing the efficacy of TENS for pain management, it is still unclear which clinical conditions should be treated with TENS and which parameters of stimulation should be used.6
The clinical literature on TENS is controversial and difficult to interpret, mainly due to study limitations and poor design. TENS-related factors (i.e., frequency, intensity, pulse duration, number of weekly or even daily sessions, interval between administrations, duration of stimulation and shape of the electrode, number of channels, as well as form and area of electrode placement) are not always specified, appropriate, or consistent among patients. Further appropriate stimulation parameters may not always be used.
A recent meta-analysis investigating the effect of TENS on postoperative pain, measured as analgesic consumption, showed a 35% reduction for TENS applied at adequate stimulation parameters (frequency: 1–8Hz for acupuncture-like TENS or 25–150Hz for conventional TENS; intensity: strong subnoxious, maximal tolerable, or >15mA). Without adequate frequency and intensity of stimulation, there was only a 4% reduction in analgesic consumption.7 Similarly, Rakel and Frantz8 found that TENS intensities >9mA resulted in greater reduction in pain during gait and vital capacity activities postoperatively compared to TENS <9mA. Thus, adequate dosing is essential to obtain a positive effect.
Electrode size, as presented by Cheing and Hui-Chan9 and Resende et al.,10 can intervene in the density of the electrical current transmitted to tissues under the electrode. With uniform electrode conductivity, the current density is inversely proportional to the electrode contact area. Therefore, as electrode contact area decreases, current density increases, meaning that if the same electrical voltage is applied first across a pair of small electrodes and then across a pair of large electrodes, the amplitude of stimulation will feel greater beneath the smaller pair. If one small and one large electrode are used in a single application, the stimulation will generally be perceived as greater beneath the small electrode.11 Thus, larger electrodes will be able to deliver more current to the tissue. Moreover, application of electrodes in a linear, parallel, crossed, or alternating design and the distance between the electrodes are often detailed in studies.8
Thus, we aimed to compare the TENS effect in different body areas in an experimental model of inflammatory pain in rats. Moreover, we intended to determine whether electrode placement influences the development of tolerance to TENS and edema reduction.
MethodAnimalsAll experiments were approved by the Animal Care and Use Committee at Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil (approval number 88/10) and are in accordance with the guidelines of the Brazilian College of Animal Experimentation and the International Association for the Study of Pain for the use of laboratory animals. Thirty adult male Wistar rats, 6 in each group as suggested for animal research (weight range, 250–300g), were kept at the Laboratory of Neuroscience Research at a temperature of 22–24°C, in a light-controlled room (12h/12h light/dark cycle, lights on at 6:00a.m.). The rats were housed with a maximum of five per cage, with water and food ad libitum.
Induction of inflammationImmediately after baseline behavioral measurements, rats were anesthetized with 5% isoflurane, maintained with 1–2%. Knee joint inflammation was then induced by intra-articular injection of a mixture of 3% carrageenan and kaolin (0.1mL in sterile saline; pH, 7.4) into the left knee joint.12 The inflammation is considered acute for the first 24h, when there is neutrophil infiltration. This model is used to mimic arthritic conditions and shows good predictability for drug effects.13
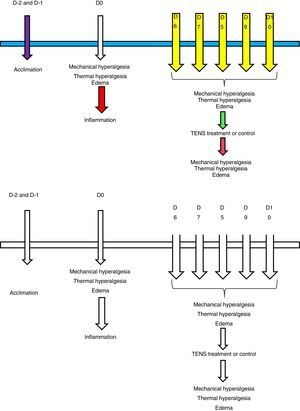
After induction of knee inflammation, the rats were returned to their cages and allowed to recover for 24h. Within 24h, the animals exhibited signs of inflammation such as edematous and warm knee joints and also behavioral signs such as guarding and decreased weight bearing on the inflamed limb.14 Within 5 days of treatment, the animals were assessed for both mechanical and thermal hyperalgesia, as well as edema volume, before and after application of TENS.
Mechanical sensitivityPaw withdrawal thresholds were tested for all groups of rats. Measurements were performed before the induction of hyperalgesia and 24h after, in order to confirm this induction, and immediately before and after TENS on each day. The researcher was blinded to all stimuli and did not participate in the application of TENS, remaining in a separate room doing the evaluations before and after treatment. We tested the rats for paw withdrawal threshold with von Frey filaments applied to the paw. Initially, the animals were acclimated in their cages for 30min in the behavior testing room. Then, animals were placed in transparent acrylic cubicles over a wire mesh and acclimated for another 30min before testing. A series of filaments with increasing bending forces (range, 9–200mN) were applied on the plantar surface of the hind paw until the rat withdrew from the stimulus.14 Each filament was applied twice. The lowest force at which the rat withdrew its paw from 1 of 2 applications was recorded as the paw withdrawal threshold for mechanical hyperalgesia and interpreted as cutaneous hyperalgesia. This testing method has shown significant test–retest reliability.15
Thermal sensitivityInitially, animals were acclimated to this experiment over two consecutive days before induction of inflammation. The test was performed with the rats in clear acrylic cages, with the tail over a heat source that was switched off. The animals were placed in the cages for three 5-min sessions, with a 30-min interval between sessions. The heat source was switched on until it reached 50°C for a maximum period of 20s. The time for the animal to withdraw its tail from the heat source was recorded and interpreted as latency to heat stimuli.15
EdemaJoint edema was measured with a caliper, which is a recognized instrument for that purpose in the literature. For this measurement, the animals were lightly anesthetized with isoflurane 5–2%).7–9 Their knee joints (both left and right) were extended and a measure was taken in the anteroposterior and mediolateral directions.
All tests were performed before and 24h after the induction of inflammation and immediately before and after the administration of active TENS or control on each day of intervention, taking advantage of the effect of isoflurane already used for treatment (see Fig. 1).
Application of TENSWe anesthetized the rats with isoflurane, initially with 5%, and maintained with 1–2% for 20min of treatment of TENS.16 It has been previously demonstrated that1 application of isoflurane without TENS has no effect on paw withdrawal latency to heat induced by joint inflammation and2 application of TENS to a non-inflamed knee joint has no effect on paw withdrawal latency.17 EMPI Select TENS units with an asymmetrical biphasic square wave and circular electrodes were used. Under isoflurane anesthesia, 1.3cm (0.5in.) pre-gelled surface electrodes were applied to the medial and lateral aspects of the inflamed knee joint in the groups receiving TENS or sham TENS. We observed the animals continuously during TENS to ensure adequate anesthesia and to ensure that the electrodes remained in contact with the skin.
We administered low-frequency TENS, keeping other parameters constant, e.g., sensory intensity and 20min of stimulation, and changing only the site of electrode placement in all the groups. This strategy allowed a comparison of different placements without confounding differences in the biophysical parameters of stimulation. Sensory intensity was determined by increasing the intensity until a muscle contraction was visibly observed and then reducing the intensity to just below this level. Parameters were similar to those used clinically and which produced full inhibition of secondary hyperalgesia.17 The control group was anesthetized with 1–2% isoflurane and electrodes were placed on their shaved knee joint, but they did not receive TENS treatment.
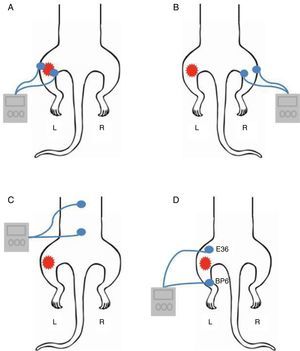
Groups of animalsTENS was actively administered to five different groups of animals (n=6 each, as suggested in the literature): (1) dermatome group – electrodes applied to the medial and lateral aspects of the inflamed knee joint18 (Fig. 2A); (2) contralateral group – electrodes placed on the contralateral knee joint (not inflamed) (Fig. 2B); (3) paraspinal group – electrodes positioned on the paraspinal muscles (L1–L5), corresponding to nerve roots that innervate the hind paw (Fig. 2C); (4) acupoint group – electrodes were placed on the “Zusanli” or Stomach acupoint (St36), located on the tibialis anterior 10mm distal to the knee joint, and on the “Sanyinjiao” or Spleen acupoint (Sp6), located above the tip of the medial malleolus18,19 (Fig. 2D); (5) control group – sham TENS with no current delivered to the skin, although the electrodes were placed on the inflamed knee joint as in the dermatome group and anesthetized for 20min. All sites that received the application of surface electrodes were shaved for better coupling with the skin.
The anesthetic procedure was the same in all groups and at the induction of hyperalgesia. The behavioral tests were performed 2h after anesthesia so as not to influence the data.
Statistical analysisThe Shapiro–Wilk test was used to verify data normality. As data followed a non-normal distribution, the Kruskal–Wallis test was used to analyze differences between groups, followed by Tukey's post hoc. For intragroup differences, the Wilcoxon matched-pairs test was used. p values less than 0.05 were considered significant.
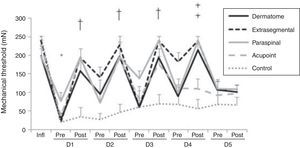
ResultsMechanical hyperalgesiaAll treatment groups showed a significant decrease in mechanical withdrawal threshold of the paw 24h following the induction of inflammation (p<0.001). There was a significant increase in mechanical withdrawal threshold up to the fourth day after TENS administration for the groups that received electrodes to the dermatome, extrasegmental, and paraspinal sites and up to the third day when acupoints were stimulated in comparison with control and pre-treatment measures (p<0.05). It is worth noting that the extrasegmental group maintained a higher threshold between pre- and post-assessment on the fourth day. On the fifth day of repeated TENS administration, none of the groups treated with active TENS showed significant differences for the mechanical withdrawal threshold when compared with control and pre-treatment measures (p>0.05) (Fig. 3).
Line graph representing mechanical withdrawal threshold (mN) of the left paw in groups whose electrodes were placed in different areas. Data are expressed as mean and standard error of the mean. Infl: before inflammation. D: Day. Pre: pre-TENS. Post: post-TENS. *p<0.001 compared to before inflammation (Wilcoxon matched-pairs test). † p<0.05 in comparison with pre-TENS (Wilcoxon matched-pairs test) and control (Kruskal–Wallis test) group. ‡ p<0.02 in comparison with pre-TENS (Wilcoxon matched-pairs test) and acupoint and control (Kruskal–Wallis test) groups.
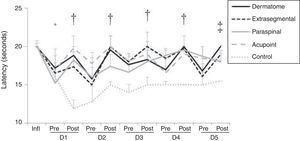
There was a significant reduction in latency to thermal stimulus in all groups 24h after the induction of inflammation (p<0.02). Compared to the control group, all other groups that received active TENS showed a significant increase in latency to react to thermal stimulus after the treatment (p<0.02). This result was observed after daily treatments on the first five days, except the group that was stimulated through the acupoint site. The latter showed no difference in latency compared to the pre-treatment measure (Fig. 4).
Line graph representing thermal latency (seconds) of the left paw in groups whose electrodes were placed in different areas. Data are expressed as mean and standard error of the mean. Infl: before inflammation. D: Day. Pre: pre-TENS. Post: post-TENS. *p<0.02 compared to before inflammation (Wilcoxon matched-pairs test). †p<0.01 in comparison with pre-TENS (Wilcoxon matched-pairs test) and control (Kruskal–Wallis test) group. ‡p<0.02 in comparison with pre-TENS (Wilcoxon Matched Pairs test) and control (Kruskal–Wallis test) group.
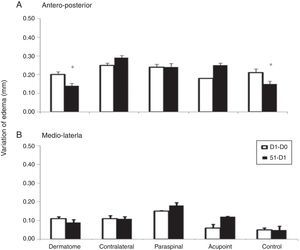
Regarding the measurement of knee edema, there was no significant difference in the perimeter of knee in the groups dermatome and control when anteroposterior measure was performed (p<0.05) (Fig. 5).
Bar graph representing variation of edema (mm) from the left knee joint in groups whose electrodes were placed in different areas. Data are expressed as mean and standard error of the mean. D0: before induction of inflammation. D1: 24h after induction of inflammation. D5: after fifth session of TENS administration. *p<0.05 in comparison with other groups (Kruskal–Wallis test).
To the best of our knowledge, this is the first study that investigated and compared the analgesic effect of TENS application by placing electrodes at different anatomical sites in animal models of inflammatory pain. The placement of the electrodes along the paraspinal muscles constitutes an alternative way to reach the receptive field related to the injury site without conducting direct application of electrodes. The present study confirms the effectiveness of TENS applied to the paraspinal region as there was a reduction of mechanical and thermal hyperalgesia in this animal model. Somers and Clemente20 administered TENS in a neuropathic pain model in rats by using self-adhesive electrodes applied to the skin innervated by the dorsal branch of the right lumbar spinal nerves (L1–L5), demonstrating that TENS administered soon after surgery nerve constriction prevented the development of thermal allodynia.
Another important outcome showed a significant increase in mechanical withdrawal threshold and latency to thermal hyperalgesia produced by TENS at acupoints Sp6 and St36. Previous studies have demonstrated that TENS applied to acupuncture points in the complex model of inflammation induced by complete Freund's adjuvant (CFA) promotes analgesic effects by raising thermal and mechanical threshold and suppresses the activation of extracellular signal-regulated kinase 2 (ERK2) and CFA-induced expression of Fos protein. In addition, both TENS and electroacupuncture (EA) promoted an increase in latency to tail withdrawal when applied at acupoints St36 and Sp6.21
Dean et al.22 investigated the effects of TENS applied over the right median nerve of the ipsilateral and contralateral limbs and found a greater ipsilateral threshold in relation to the contralateral in all sensory modalities, although the differences were less marked for thermal stimulus. In agreement with our results, a study that induced pain in humans using the tourniquet technique obtained similar analgesia by administering TENS at the site of ischemic pain (ipsilateral forearm) or at a site unrelated to pain (contralateral leg).23 Therefore, our results show that even when the electrodes were applied contralaterally to the inflamed knee, both the thermal and mechanical hyperalgesia were reversed after electrical stimulation, pointing out that TENS may be efficacious even if the electrodes are placed contralaterally or distant to the painful area.
In short, we found that TENS stimulation to different sites activates different somatotopic areas and promotes similar levels of anti-hyperalgesia regardless of the site of electrode placement for TENS stimulation. Previous reports have shown the involvement of opioidergic, serotoninergic, and GABAergic pathways in TENS-induced analgesia and its importance for the inhibition of pain at supraspinal and spinal levels. The inhibitory neurotransmitter GABA, but not glycine, is involved in the analgesic effects by TENS in the spinal cord. Both low- and high-frequency TENS produce analgesia through activation of endogenous opioid pathways.24,25 Specifically, spinal and supraspinal (RVM) administration of either delta- (naltrindole) or mu- (naloxone) opioid-receptor antagonists prevent analgesia induced by high- and low-frequency TENS, respectively.26,27 High-frequency TENS increases extracellular GABA concentration in the spinal cord in animals with and without joint inflammation. Interestingly, the blockade of GABA receptors prevents the reduction in hyperalgesia produced by high- and low-frequency TENS.28
Recently, our group showed that the periaqueductal gray (PAG) also contributes to the TENS-induced analgesic effect. Antinociception produced by high- and low-frequency TENS is prevented by blocking the ventrolateral PAG with cobalt chloride. Thus, the PAG sends projections to the rostral ventrolateral medulla (RVM), resulting in analgesia.29
In conditions that cause persistent pain, such as inflammation or injury, the function of descending pathways may change considerably. In contrast, depending on a number of factors, injury and inflammation may produce a reduction in descending inhibition or an increase in descending facilitation of pain. The function of descending pain-inhibitory systems may be enhanced by some centrally acting therapies (which act on the monoaminergic system or opioid receptors), direct stimulation of brain areas involved in descending inhibitory controls, indirect activation of descending pathways with peripheral stimulation, or behavioral manipulations.30
Our findings do not allow us to determine whether there was a reduction in the volume of edema after application of TENS; however, they corroborate the research developed by Resende et al.10 These authors found no significant changes after the application of high- and low-frequency TENS in an animal pain model identical to that developed in our study.
Interestingly, it was possible to detect the loss of the effectiveness of TENS in reversing mechanical and thermal hyperalgesia on the fifth day, suggesting the development of the phenomenon of tolerance. Analgesic tolerance to TENS was first demonstrated by Chandran and Sluka.31 After repeated application of successive high- and low-frequency TENS, there was a significant loss of effectiveness in promoting anti-hyperalgesia due to analgesic tolerance at spinal opioid receptors.
Similarly to N-methyl-d-aspartate (NMDA) receptor antagonists, intrathecal and systemic administration of cholecystokinin (CCK) agonists inhibits the development of tolerance to TENS application on the fourth day, when administered prior to application of TENS on the first three days, and prevents cross-tolerance at opioid receptors. In addition, modulating frequency TENS between high and low also slows the development of analgesic tolerance to TENS,32 as did the association between frequency variation and increase in stimuli intensity.33 Thus, the site of application of the electrodes during stimulation with TENS also did not seem to affect the development of tolerance to the anti-hyperalgesic effects promoted by TENS.
ConclusionTENS electrodes applied at different sites on the body promote the same level of anti-hyperalgesic effect, suggesting the action of TENS in the central nervous system and possibly involving descending inhibitory pathways. This may be of particular interest in private practice as it allows different sites for TENS application in cases of clinical pain conditions such as burns and neuropathic pain. This study also showed no effect on reducing edema, but the results suggest the occurrence of analgesic tolerance to TENS.
Conflicts of interestThe authors declare no conflicts of interest.