To evaluate the stability, postural adjustments and contributions of sensory information for postural control in children.
Methods40 boys and 40 girls were equally divided into groups of 5, 7, 9 and 12 years (G5, G7, G9 and G12). All children were submitted to dynamic posturography using a modified sensory organization test, using four sensory conditions: combining stable or sway referencing platform with eyes opened, or closed. The area and displacements of the center of pressure were used to determine stability, while the adjustments were used to measure the speed of the center of pressure displacements. These measurements were compared between groups and test conditions.
ResultsStability tends to increase with age and to decrease with sensory manipulation with significant differences between G5 and G7 in different measures. G7 differed from G12 under the conditions of stable and sway platform with eyes open. G9 did not differ from G12. Similar behavior was observed for adjustments, especially in anterior–posterior directions.
ConclusionPostural stability and adjustments were associated with age and were influenced by sensory manipulation. The ability to perform anterior–posterior adjustments was more evident and sensory maturation occurred firstly on the visual system, then proprioceptive system, and finally, the vestibular system, reaching functional maturity at nine years of age. Seven-year-olds seem to go through a period of differentiated singularity in postural control.
The acquisition of postural control is multifactorial. Despite the fact that postural control has been investigated in two studies,1,2 sensory mechanisms of postural control are still unclear in literature. Besides the interactions between sensory information and motor performance in children, postural control also depends on the maturation of the structures involved, as well as on their motor experiences. Furthermore, in order to maintain postural control, the central nervous system makes differential use of sensory information during various phases of motor development.1,2
By the age of seven years, the development of the structures responsible for motor control is complete,3 and studies agree that seven-year-old children have generally achieved mature postural control3–6 as evaluated by displacements of the center of pressure (COP). However, children at this age have not yet had enough motor experiences for their complete motor development and postural control7 and require skill development for postural adjustments.
According to Roncesvalles et al.,8 by the age of 10 years, children will have achieved postural stability similar to adults. However, postural control, as determined by the speed of COP displacements, is generally developed during puberty.6 There have been reports that children between the ages of four and five years of age employing large and rapid postural adjustment strategies.9 From 8 to 10 years of age, reductions in speed and increases in precision of adjustments are clearly seen, as there is better integration between the open and closed-loop control systems to maintain this control.6,9,10 In addition, amongst biomechanical parameters, the COP speed displacements appear to best characterize postural control.11 Thus, there is a need to standardize postural control variables for various ages, so that the results are more conclusive.
Another issue that must be clarified is the importance each type of sensory information has in postural control during child development. The predominance of one sensory system may be a strategy adopted by the nervous system to avoid information conflicts,12 and may vary according to age, motor experiences, and system maturation. Up to the age of 11 years, visual information appears not to have the same importance for postural control as it does in adults, and vestibular information is integrated only after the age of 12 years.13,14
The aim was to compare postural control through postural stability and adjustments in children aged 5–12 years, and the contribution of sensory information to postural control within each age group.
MethodsStudy type and participantsThis was a cross-sectional study that recruited a non-probabilistic convenience sample of healthy school children (40 boys and 40 girls) with no neurological or musculoskeletal disorders from a large metropolitan area in Brazil.15 Children were recruited at local elementary schools of different social levels. All children took part in physical education classes, and were deemed active. The subjects were equally divided into four groups according to age (G5, G7, G9, and G12). Each group consisted of 10 boys and 10 girls. The sample size calculation using a power of 0.8 estimated 10 children would be needed in each age group This study was approved by the Research Ethics Committee of Universidade Federal de São Paulo (UNIFESP), Santos, SP (protocol 128/11) Board of each institution, and consent was obtained from all parents.
Measuring instrumentsA double-force platform with transducers and amplifiers, connected to a computer, was used to perform the tests. The commands of the sway mechanism were provided by the Pro Balance Master software (8.1.0) (NeuroCom Inc., Oregon, USA). A modified sensory organization test was used for the computerized dynamic posturography – a non-invasive procedure to determine postural balance performance in different sensory conditions. This test assessed balance together with sensory visual conditions (i.e. open and closed eyes) and support surfaces (i.e. fixed and sway referencing platforms).
The sway referencing platform condition was obtained using the servo-driving mechanism, which promoted proportional rotations in the platform for anterior–posterior oscillations. Through this sway referencing platform, the Achilles tendon lengthening reflex was altered, the information to the surface of the platform was reduced, and a situation of sensory conflict was induced. The visual information was manipulated for the closed-eye condition. The condition of the closed-eye sway-referencing platform was considered to be the predominance of vestibular information for postural control.
ProceduresThe children were assessed barefoot, wearing comfortable clothes, and were supervised by an examiner because of the risk of falling. The assessment was performed at a laboratory in the Psychiatric Institute of the University of São Paulo, a well-lit and ventilated room with adequate heat and sound. The children were instructed to stand on the platform with their arms by the sides of their body. Their feet were positioned and corrected by the examiner, according to the standardization processes for the platform. When in position, three trials of 20s were performed at a sampling frequency of 100Hz for the four test conditions, in the following order: open-eye, fixed-platform (FIX/op); closed-eye, fixed-platform (FIX/cl); open-eye, sway-referencing-platform (SWR/op); and, closed-eye, sway-referencing-platform (SWR/cl).
Data processingThe COP coordinates were calculated from the force platform. The Y and X-axes indicated the anterior–posterior and medial-lateral directions, respectively. For data processing, the first trial for each test condition was discarded to allow for familiarization. The COP parameters, as a function of time, were calculated for the other two trials, as follows: (1) area (cm2), as determined by the area of the ellipse, which contained 80% of the COP displacements; (2) COPX, which corresponded to the total COP displacements in the medial-lateral directions in cm; (3) COPY, as determined by the total COP displacements in the anterior–posterior directions in cm; (4) MVX, the mean velocity of the COP displacements in the medial-lateral directions in cm/s; and, (5) MVY, the mean velocities of the COP displacements in the anterior–posterior directions in cm/s.
Sensory ratios were calculated by the closed-eye fixed-platform condition with the open-eye fixed-platform (baseline) one, yielding somatosensory ratios; the open-eye sway-referencing-platform condition with the open-eye fixed-platform one, yielding visual ratios; and the closed-eye sway-referencing-platform with the open-eye fixed-platform one, yielding vestibular ratios, as proposed by the Pro-balance Master software.
Statistical analysisRepeated-measures ANOVAs, followed by planned contrasts (Tukey test), were used to investigate the differences in postural control parameters amongst the test conditions. Two factors were considered in the model: test conditions (FIX/op; FIX/cl; SWR/op, and SWR/cl) as intra-subject factors, and groups (G5, G7, G9, G12), as inter-subject factors. The assumptions for the employment of these tests were verified through Mauchly tests and graphic representations of the normal probability of the residues. Finally, repeated-measures ANOVAs were used to calculate the differences in sensory ratios: somatosensory, visual, and vestibular, followed by planned contrasts, using a Tukey test. One factor was considered in the model: groups (G5, G7, G9, G12). The significance level of all tests was set at α<0.05.
ResultsSubject height, body mass, body mass index (BMI), and BMI percentile are shown in Table 1. Postural control parameters were presented in relation to stability (i.e. areas and COP displacements) and to the adjustments (i.e. COP velocity), through the comparison of sensory manipulation conditions throughout the age groups.
Mean (±SD), minimum and maximum values (min–max) for height, mass and body mass index (BMI) and BMI percentile in each age group of children used in the study. Each age group contained 10 girls and 10 boys. G5 (five years old), G7 (seven years old), G9 (nine years old) and G12 (12 years old).
| G5 | G7 | G9 | G12 | |
|---|---|---|---|---|
| Height (m) | ||||
| Mean (SD) | 1.12 (±0.04) | 1.23 (±0.05) | 1.35 (±0.07) | 1.53 (±0.1) |
| Min–max | 1.08–1.22 | 1.17–1.42 | 1.25–1.46 | 1.34–1.73 |
| Mass (kg) | ||||
| Mean (SD) | 20.05 (±2.91) | 26.8 (±3.30) | 33.80 (±7.8) | 46.6 (±7.9) |
| Min–max | 16–26 | 21–44 | 22–48 | 26–67 |
| BMI | ||||
| Mean (SD) | 15.8 (±1.5) | 18.32 (±4.1) | 18.7 (±3.4) | 20.6 (±4.9) |
| Min–max | 13.7–18.3 | 14.3–29.6 | 13.8–24.1 | 13.5–28.6 |
| BMI percentile | P85 | P85 | P95 | P85 |
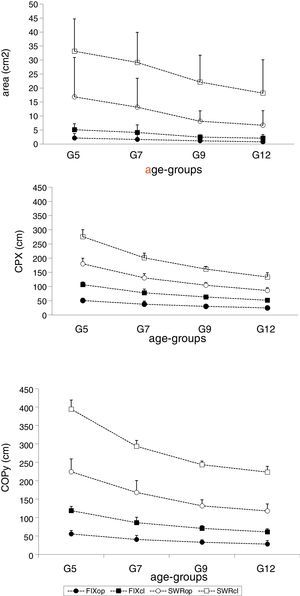
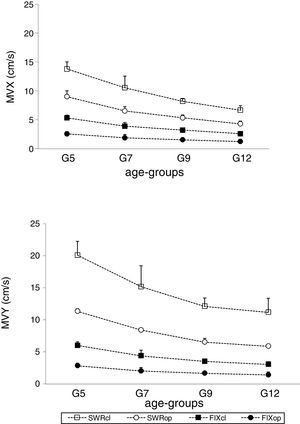
Figs. 1 and 2 depict the means and standard deviations of the stability and adjustment parameters, considering testing conditions and age groups. It is noticeable that the mean values increased with postural demands due to sensory manipulations, and decreased with age. Increase in postural demands has been considered changes for testing conditions FIX/op; FIX/cl; SWR/op; and, SWR/cl.
Comparison between children of 4 age differences (5, 7, 9, and 12 years of age) with respect to (1) area delimited by the center of pressure (COP) in cm2 (AREA); (2) maximum displacement of COP in X-axis or latero-lateral direction (COPX) in cm; (3) maximum displacement of COP in Y-axis or antero-posterior direction (COPY) in cm. Error bars=SD. Conditions: FIX/op; FlX/cl; SWR/op and SWR/cl.
COP area oscillations increased with difficulties in postural demands (F=73.693, df=1.890, p<0.001), but these differences depended on the age group (F=2.468, df=3, p=0.068). Independent of age, COP mean area oscillations increased with greater postural demands. Fig. 1 shows that the reduction in COP area oscillations associated with age advance was more evident for the conditions with higher postural demands – SWR/op, and SWR/cl.
COP displacements in medial-lateral and anterior–posterior directionsBoth medial-lateral (COPX) (F=186.402, df=1.747, p<0.0001) and anterior–posterior (COPY) (F=314.011, df=1.709, p<0.001) displacements increased with greater postural demands (Table 2); and varied between age groups for the COPX (F=50.445, df=3, p<0.001) and for the COPY (F=25.953, df=3, p=0.001) (Fig. 1 and Table 3).
Mean differences, 95% CI (lower bound and upper bound) and significant differences (p-value) in the test conditions (FIX/op; FlX/cl; SWR/op and SWR/cl) for the sensory organization test (Nashner et al., 1982).16 Variables: COPX, COPY, MVX and MVY.
| Test conditions | COPX | COPY | MVX | MVY | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC | IC | IC | IC | |||||||||||||
| Mean difference | Lower bound | Upper bound | p | Mean difference | Lower bound | Upper bound | p | Mean difference | Lower bound | Upper bound | p | Mean difference | Lower bound | Upper bound | p | |
| FIX/op×FIX/cl | −2.675 | −4.297 | −1.054 | 0.0001* | −5.030 | −7.172 | −2.889 | 0.0001* | −0.133 | −0.217 | −0.050 | 0.0001* | −0.263 | −0.398 | −0.128 | 0.0001* |
| FIX/op×SWR/op | −14.171 | −17.086 | −11.256 | 0.0001* | −36.528 | −43.618 | −29.437 | 0.0001* | −0.720 | −0.871 | −0.569 | 0.0001* | −1.795 | −0.2131 | −1.459 | 0.0001* |
| FIX/op×SWR/cl | −31.347 | −36.354 | −26.340 | 0.0001* | −88.495 | −99.667 | −77.323 | 0.0001* | −1.688 | −2.080 | −1.296 | 0.0001* | −4.604 | −5.306 | −3.902 | 0.0001* |
| FIX/cl×SWR/op | −11.496 | −14.506 | −8.485 | 0.0001* | −31.497 | −38.184 | −24.811 | 0.0001* | −0.586 | −0.742 | −0.430 | 0.0001* | −1.532 | −1.834 | −1.230 | 0.0001* |
| FIX/cl×SWR/cl | −26.672 | −33.596 | −23.748 | 0.0001* | −83.465 | −94.305 | −72.624 | 0.0001* | −1.555 | −1.946 | −1.164 | 0.0001* | −4.341 | −5.045 | −3.637 | 0.0001* |
| SWR/op×SWR/cl | −17.176 | −22.330 | −12.023 | 0.0001* | −51.967 | −63.014 | −40.920 | 0.0001* | −0.969 | −1.337 | −0.600 | 0.0001* | −2.809 | −3.490 | −2.129 | 0.0001* |
Mean differences, 95% CI (lower bound and upper bound) and significant differences (p-value) in the group (age) for the sensory organization test for children aged 5, 7, 9 and 12 years old (Nashner et al., 1982).16 Conditions: FIX/op; FlX/cl; SWR/op and SWR/cl. Variables: COPX, COPy, MVX and MVY.
| Test conditions | COPX | COPY | MVX | MVY | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC | IC | IC | IC | |||||||||||||
| Mean difference | Lower bound | Upper bound | p | Mean difference | Lower bound | Upper bound | p | Mean difference | Lower bound | Upper bound | p | Mean difference | Lower bound | Upper bound | p | |
| G5×G7 | 18.602 | 10.511 | 26.6931 | 0.0001* | 25.297 | 11.391 | 39.202 | 0.0001* | 0.811 | 0.353 | 1.269 | 0.0001* | 1.228 | 0.494 | 1.961 | 0.0001* |
| G5×G9 | 28.606 | 20.515 | 36.697 | 0.0001* | 37.701 | 23.796 | 51.607 | 0.0001* | 1.396 | 0.925 | 1.867 | 0.0001* | 1.993 | 1.2595 | 2.727 | 0.0001* |
| G5×G12 | 35.612 | 27.525 | 43.707 | 0.0001* | 42.6797 | 28.774 | 56.585 | 0.0001* | 1.781 | 1.323 | 2.239 | 0.0001* | 2.230 | 1.496 | 2.964 | 0.0001* |
| G7×G9 | 10.004 | 1.913 | 18.095 | 0.009* | 12.405 | −1.500 | 26.310 | 0.097 | 0.585 | 0.114 | 1.055 | 0.009* | 0.765 | 0.031 | 1.499 | 0.038* |
| G7×G12 | 17.014 | 8.923 | 25.1052 | 0.0001* | 17.383 | 3.477 | 31.288 | 0.008* | 0.970 | 0.512 | 1.428 | 0.0001* | 1.002 | 0.268 | 1.736 | 0.003* |
| G9×G12 | 7.009 | −1.081 | 15.1004 | 0.113 | 4.9778 | −8.9278 | 18.883 | 0.783 | 0.3855 | −0.0853 | 0.8563 | 0.147 | 0.236 | −0.497 | 0.970 | 0.831 |
Multiple comparisons revealed that G5 demonstrated greater medial-lateral and anterior–posterior displacements than G7, G9, and G12 for all postural demand conditions (Tables 2 and 3). COPY and COPX displacements were greater for G7 when compared to G12 only for the conditions FIX/op (p=0.0002) and FIX/cl (p=0.0028), and COPX displacement for SWR/op (p=0.0213). No significant differences were found between G9 and G12 for any of the investigated parameters (Table 3).
Postural adjustmentsVelocity of COP displacements in medial-lateral and anterior–posterior directionsThe mean velocities of COP displacements in both medial-lateral (MVX) (F=104.894, df=1.326, p<0.001) and anterior–posterior (MVY) (F=234.824, df=1.414, p<0.001) directions showed a significant increase with higher postural demands (Table 3), but this increase depended on the age group for MVX (F=39.200, df=3, p=0.0001) as well as for MVY (F=25.843, df=3, p=0.0001) (Fig. 2 and Table 3).
Multiple comparisons revealed that the G5 group performed medial-lateral and anterior–posterior adjustments faster than G7 for FIX/op and FIX/cl (Tables 2 and 3), and anterior–posterior adjustment in SWR/op and SWR/cl (Table 2). However, between G7 and G9, these differences only occurred for the FIX/op condition in medial-lateral adjustments (p=0.052).
Regarding anterior–posterior adjustments, multiple comparisons revealed that the G7 group performed anterior–posterior and medial-lateral adjustments significantly faster than G12 for the conditions FIX/op (p=0.0002) and FIX/cl (p=0.0028), and anterior–posterior adjustments for the SWR/op condition (p=0.021). No significant differences were observed between the G9 and G12 groups regarding postural adjustment parameters.
The somatosensory ratio did not show significant differences between the groups. The visual ratio was significantly different between groups (F=4.0; df=3; p<0.011). The contrast analysis showed differences between the G5 and G12 groups (p=0.009). The vestibular ratio differed significantly between groups (F=9.01, df=3, p<0.0001). The contrast analysis showed differences between the G5 and G9 groups (p=0.05), G5 and G12 groups (p=0.0001), and G7 and G12 groups (p=0.001).
DiscussionThis study on the postural control of children from 5 to 12 years of age showed that postural oscillations decreased with age and increase with postural demands, indicating control of multisensory reweighting. The present results revealed similar behaviors for all other variables. Research distinguishes COP-based measurements related to body sway frequency showing below 0.5–1.0Hz related conditions when dominant visual-vestibular sensory information is above 0.5–1.0Hz in relation to somatosensory dominance conditions.17,18
Displacement changes in both directions may have reflected the maturation of neuromuscular mechanisms involving sensory and muscular processes. However, the COP displacements in the present study were higher for G5, when compared to those in G7 for all sensory manipulation conditions. For the seven years olds, in postural conditions with lower demands of open and close eyes, fixed-platform condition, COPX displacements tended to be greater than those in the G9 group. For higher postural demands, with close-eyes, sway-referencing-platform, seven and nine year olds showed the same levels of control difficulty for medial-lateral and anterior–posterior displacements. This suggested that postural control developed more rapidly in these directions than in the medial-lateral direction, due to gait development, which matures early in childhood.19
Five year olds performed quicker postural adjustments in anterior–posterior directions, when compared to seven year olds, for all sensory manipulation conditions. For medial-lateral adjustments, these differences in speed only occurred for the easiest conditions (i.e. when the platform was fixed). Similarly, the stability parameters for the G7 group, postural adjustments were faster than those for the G9 group only in the medial-lateral direction for the fixed open-eye test condition. This indicated that G7 was learning to adjust to lower demands. This finding showed that sensory integration happened later for postural adjustment, as the G5 and G7 groups used high oscillation velocity in both directions, which could predispose the child to postural instability. On the other hand, no differences were found between the G9 and G12 groups.20
According to the literature, children aged 12 do not show the same levels of performance during the maintenance of the standing position as adults do, as they still have some difficulty in estimating spatial body positions and, consequently, the functioning of the closed-loop control mechanisms still differs from those in adults.21–23 The present findings showed that children aged nine and 12 had similar postural adjustment behaviors for still-standing posture and also for all other sensory manipulation conditions. Based on these findings, it is possible to argue that some levels of motor control maturation might occur before the age of 12. This behavior reflects the functional maturation of the integrated motor and sensory systems, as seen when changes in sensory input could modify motor response during a task.24 Such findings corroborated previous reports of differences in the functioning of the balance control system, which increase with age.2,4,5,9,25,26 Meanwhile, the present study provided deeper and more comprehensive knowledge of postural control in children, based on sensory cues.
The maturation of sensory systems has influence on postural control. Thus, the use of sensory information is dependent on age. Visual system is the first sensory system to mature; our results showed that by the age of five years, it was fully ready, as response patterns were similar for all ages. This finding agreed with that of the moving room, when the child started to balance in the sway-referenced visual by age 4.27 Therefore, children aged five are adaptable to changes in the visual stimuli. This information could be used in clinical practice for training postural control through abrupt changes in visual cues. Until the age of seven, children are unable to properly solve conflicting sensory information situations; despite the fact that the coupling of sensory information and postural control started in infancy, sensory cues provide conflicting information, challenging postural control in these children. Bortolaia et al.28 discussed that by the age of seven, children tended to have less postural control, with smaller magnitude and variability of this behavior due to changes in the sensory systems integration processes. Another aspect referring to changes in postural control in children aged seven years is the growth spurt observed during that age, due to morphological changes and postural control.25,29
Children aged seven years could stabilize visual and somatosensory inputs, but they were incapable of controlling vestibular inputs. Moreover, it appeared that by age 9, children integrated sensory cues from different sources for postural control, as seen in the closed-eye sway surface condition, when vestibular information was used because somatosensory cues were not precise enough for postural control functioning.30 This behavior reflected functional maturation of the integrated motor and sensory systems, as it happened when changes in sensory inputs could modify motor response for a given task.24 On the other hand, the results showed that children aged nine years used vestibular information in a more evident way, because the response pattern from postural control did not differ between the ages nine and 12 years, indicating that from that age, it was possible to integrate sensory inputs from different sources.
It is well known that sensory information plays an important role in postural control and, when it is associated with the control of voluntary movements, it facilitates a broad understanding of motor action control. The functional maturation of the sensory integration system appeared to occur at nine years of age, a time when children can respond to more than one sensory cue.27 The presence of pathological conditions may lead to the inability to employ this information, which is necessary for motor control. This information, which is used for postural adjustments, resulted in modified structures over time and throughout the learning process, and it is often employed in rehabilitation programs.
Balance control is a complex sensory-cognitive-motor task, and it challenges our skills to maintain the reference for static and dynamic posture. The unique sensory information load gives us an idea of redundancy and similarity of what the nervous system can feel and perceive. Thus, the features of predominance and individuality of each sensory system helps one understand individuality and preference, depending on age. Additionally, it can highlight that the singularity of the balance control of a child is paramount for children, and that pathologies can add more features to this unique case. In a similar way, training balance control in children can demand specific clinical evaluation of the different sensory systems in functional view, to guide specific training such as visual, somatosensory, or vestibular exercises. We can develop steps for sensory information that is less active, or train with information that is more active. It all depends on the child's balance control, the assessment of the pathology when it is present, the motor or sports training, and some skills. Altogether, it can lead to specific exercises, sports, or treatment approaches.
ConclusionsPostural stability and adjustments were associated with age and were influenced by sensory manipulation. The ability to perform anterior–posterior adjustments was more evident and sensory maturation occurred in the visual system first, followed by proprioceptive, and finally, the vestibular system, reaching functional maturity at nine years of age. Seven-year-olds seem to go through a period of differentiated singularity in postural control.
Conflicts of interestThe authors declare no conflicts of interest.