There is evidence of hypertensive effects caused by anabolic androgenic steroids (AAS). A single exercise session promotes the acute reduction of blood pressure, but the effects of AAS on this phenomenon are unknown.
ObjectivesTo investigate the post-exercise blood pressure response in androgenic-anabolic steroid users.
MethodsThirteen AAS users (23.9±4.3 years old) and sixteen controls (22.1±4.5 years old) performed a session of aerobic exercise. Heart rate and blood pressure were assessed before exercise and during a 60min post-exercise resting period. Repeated ANOVA measures were used to determine differences between the groups.
ResultsWhile the control group had a significant reduction in post-exercise systolic blood pressure of up to 13.9±11.6mmHg at 40min, this phenomenon was limited among AAS users who reached a maximum of 6.2±11.5mmHg at 60min. The between groups comparison revealed significant higher post-exercise hypotension (PEH) for the control group at 30min (−12.9±14.1mmHg versus −2.9±7.6mmHg), 40min (−13.9±11.6mmHg versus −2.5±8.3mmHg), 50min (−13.9±13.9mmHg versus −5.0±7.9mmHg) and 60min (−12.5±12.8mmHg versus −6.2±11.5mmHg). There was no significant diastolic PEH in any of the groups.
ConclusionsThis study demonstrated impaired systolic post-exercise hypotension as a new adverse effect of AAS usage.
A meta-regression of 187 studies to assess the overall prevalence of anabolic-androgenic steroids (AAS) concluded that non-medical AAS use is a serious, widespread public health problem that has a high prevalence in different populations. The prevalence rate was significantly greater among men (6.4%, p<0.001), in people from the Middle East (21.7%), in recreational sportsmen (18.4%, p<0.001) and in teenagers aged up to 19 years (2.5%).1
According to health organizations,2 AAS have several side effects, including cardio-metabolic disorders.3 Phenomena such as dyslipidemia, systemic inflammation, oxidative stress, vascular dysfunction, angiogenesis inhibition and increased autonomic sympathetic nervous activity4–6 have been reported among AAS users. All these events contribute to the increase in arterial pressure.7,8 Studies indicate that not only do AAS users present arterial pressure (AP) levels higher than those of the control group, but such values are also compatible with those diagnosed in patients with hypertension, despite being young and having no diseases.9,10
On the other hand, it is well established that physical training is an effective method of anti-hypertensive treatment.11 A single exercise session can promote a decrease in blood pressure levels immediately after it is over, and this effect endures for many hours—a phenomenon called post-exercise hypotension (PEH).12 The mechanisms involved are sympathetic reduction, nitric oxide production and reduction of volemia.12–14 Coincidentally, such mechanisms may be affected in AAS uses.3 Consequently, we raised the hypothesis that the PEH phenomena may occur differently in AAS users. Considering that there are no studies that answer this question, the present study was designed to investigate the effect of an aerobic exercise session in the post-exercise blood pressure response in AAS users.
MethodsParticipantsThis is a case–control study in which the case was bodybuilder practitioners, AAS users and the control was bodybuilder practitioners non-AAS users. We considered a previous study in which an aerobic exercise session promoted a decrease of 13±1mmHg in the systolic pressure in young normotensive adults,14 which resulted in an effect size of 2.9. Adopting a statistical power of 0.90 and an α error of 0.05, a minimum sample size of only four participants was found to be required for the study. Thirteen AAS users and sixteen controls were recruited. They performed resistance exercises for bodybuilding competitions at the professional level. The individuals trained at least five times a week and had been practicing this sport for at least two years, along with aerobic exercises, but no more than once per week. The AAS group has been using AAS at a baseline of at least 1 year.
None of the participants of the study presented with any known heart diseases or hypertension and did not use anti-hypertension medicines or nutritional supplements (thermogenic). No smokers were included as volunteers for the study. Participants who had difficulties performing the aerobic exercise session were not included in the study.
This study was approved by the ethics committee of the Universidade Federal do Piauí (UFPI), Teresina, PI, Brazil under protocol 07726613.6.0000.5214. All participants signed the consent form according to resolution 196/96 of the National Health Council.
ProceduresAfter one week of familiarization consisting of two sessions of aerobic exercise, individuals were anthropometrically evaluated and instructed to refrain from exercises for 48h before the experiment procedures and to avoid the intake of protein-rich foods, caffeine and alcohol during this period.
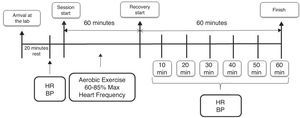
The experimental design is shown in Fig. 1. The participants performed an aerobic exercise session. Blood pressure measurements were taken before and during a post-exercise 60min period of recovery, at intervals of 10min between each measurement. The heart rate was recorded at the same time as the blood pressure and during the workout for the intensity control. All procedures were performed between 2 pm and 4 pm.
The blood pressure was evaluated according to the recommendations of the Brazilian Guidelines on Hypertension (2010),15 using a BR A100 Plus oscillometric device (MICROLIFE BR3BTO-A/BR), previously validated by Cukson et al.16 The measurements were performed by a trained and experienced evaluator. When the participants reached the laboratory for the experimental sessions, they were asked to remain seated for at least 20min in a quiet environment with a temperature between 24°C and 27°C. Subsequently, the two arm measurements were made. If there was no difference between the results, the right arm was determined for the experimental measures. If there was more than a 5-mmHg difference, the arm with the highest result was used for the procedures. Afterward, three measurements determined the blood pressure data at rest, and the two closest values were considered. After the experimental exercise session, the volunteers were instructed to sit down immediately. Then, measures in triplicate were made every 10min during a period of 60min post-exercise.
The exercise protocol consisted of 60min of running/walking with an intensity between 60% and 85% of the estimated maximum heart rate. First, the exercise began with a warm-up run on a treadmill (ProAction BH Fitness, made in Puerto del Carmen, Spain) with a duration of 3min and spontaneous intensity, considered mild by the volunteers. Immediately after warm up, they started a gradual increase in speed every minute, during the first 5min until the prescribed intensity was reached, and they remained in this intensity range until the end of the workout.
The heart rate was measured with a heart rate monitor V800 (Polar® Electro Oy, Kempele, Finland). For pre- and post-exercise measures, the volunteers remained seated. During the exercise, the measures were reported to the volunteers, and the speed of the treadmill was changed when the heart rate was not compatible with that previously established for each volunteer.
Statistical analysisThe data are presented as the mean±standard deviation of the mean. The normality and homogeneity of the data variance were analyzed via Shapiro–Wilk and Levene tests, respectively. Independent Student's t-test was used to verify differences between initial group characteristics (age, body mass index, systolic blood pressure, diastolic blood pressure and heart rate). Repeated ANOVA measures were used to compare the delta systolic and diastolic blood pressure and heart rate (dependent variables) values between groups using time and group factors. The significance level was established at p<0.05. The entire statistical analysis was performed using the SPSS version 20.0 statistical software (SPSS, Inc., Chicago, IL, USA).
ResultsThe participants in the AAS and control groups were statistically similar in age (23.9±4.3 and 22.1±4.5, respectively), whereas the body mass index (BMI) of the AAS group (27.2±2.2kg/m2) was significantly greater than that of the control group (24.7±3.1kg/m2, p<0.02). Both groups had statistically similar systolic and diastolic resting blood pressures (123.7±10.6mmHg and 74.2±11.5mmHg for AAS and 127.1±14.9mmHg and 73.8±11.3mmHg for the control), but the AAS group had a statistically higher resting heart rate (83.1±9.5bpm) than did the control group (72.3±7.8bpm, p<0.01).
The subjects reported that they had been using AAS for a minimum of two years and for a maximum of three years. All participants were at least in the 3rd, 4th or 5th cycle of their lives; the maximum was 10 cycles. They reported spending between three and four months between each cycle and undergoing between two and three cycles per year. The substances used in the current cycle were Dianabol (four users, 10–50mg/week), Deca-Durabolin (three users, 150–1000mg/week) and Durateston (seven users, 250–300mg/week), propionate/enanthate of testosterone (two users, 300mg/week), and oxandrolone (one user, 100mg/week). Six users were using only one drug in the current cycle, four were using two drugs, and three were using three drugs. They were in the current cycle for 11±3 weeks.
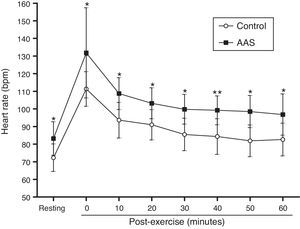
The AAS group presented a significantly higher resting heart rate than did the control group (p<0.01). They finished the exercise session with a significantly higher heart rate than did the control group (p<0.01) and showed higher values during recovery, as can be observed in Fig. 2.
Behavior of the heart rate during the 60min of post-exercise recovery. Data are presented as the means and standard deviation of the means. Response of the heart rate before and at 0, 10, 20, 30, 40, 50 and 60min post-exercise (○) control group (■) AAS group; *significant differences between groups (two-way repeated ANOVA measures, p<0.05).
While a significant decrease in the arterial pressure was observed from 30min to 60min after exercise in the control group in relation to the pre-exercise systolic pressure levels (Fig. 3, panel A), in the AAS group, there was no significant reduction during the 60min after exercise. The PEH of the control group was already 8.1±15.2mmHg at 20min after exercise and reached 13.9±11.6mmHg at 40min after exercise. Adversely, the AAS group had only 3.0±14.6mmHg at 20min after exercise and reached the maximum level of only 6.2±11.5mmHg at 60min after exercise. As shown in Fig. 3 (panel A), the PEH levels of the control group were significantly greater than those of the AAS group at 30, 40, 50 and 60min after exercise.
Variation in the systolic (panel A) and diastolic blood pressure (panel B) in relation to the resting blood pressure after one session of aerobic exercise. Data are the delta of the means and standard deviation. *Statistical within-group differences at every moment (two-way repeated ANOVA measures, p<0.05).
Neither the control group nor the AAS group had a significant diastolic PEH at any of the post-exercise measurements. Furthermore, there was no difference between groups for any of the measurements. Nonetheless, Fig. 3 (panel B) shows that the diastolic pressure variations in the control group were always negative, whereas there was a hypertensive response in descriptive terms in the AAS group at 20, 30 and 40min after exercise.
DiscussionThe systolic response in the participants of the control group corroborates other studies that adopted a similar protocol.14,17,18 Teixeira et al.,14 for example, showed that PEH occurred in men and women with an average age of 26 years (which is similar to the age of the volunteers of this study), who exercised at 75% of maximal oxygen consumption-VO2max (an intensity that is also similar to that in our study). The magnitude of the systolic PEH found in the control group (adults with a normal blood pressure) was too similar to that of hypertensive subjects.19 The diastolic hypotension data found in the present study are also compatible with the results of previous investigations, which indicate less diastolic PEH in relation to the systolic component or even an absence in participants with hypertension and normal blood pressure, who presented systolic PEH.
In the previous studies, the hypotensive response is so evident that the effect size reaches values close to 3.0, indicating a great potential for the exercise to promote systolic PEH. Nevertheless, PEH did not occur among AAS users, even considering that the sample size was much larger than that calculated, based on this effect size. Until the present study, reports indicated an increased value in the resting blood pressure in AAS users compared with non-users.20,21 To the best of our knowledge, this study is the first to demonstrate that, in addition to a higher blood pressure at rest, AAS users also fail to respond to an aerobic workout with PEH.
The BMI of users may have been an intervening factor in the response pressure after exercise. However, the relevant evidence is controversial. Brito et al.18 performed a literature review and reported that elderly people with a higher BMI have a greater reduction in cardiac output, which is a mechanism involved in PEH. Conversely, in this same review, overweight people had less reduction in peripheral resistance after exercise than did normal-weight individuals. Contrary results were also found by Viegas et al.,22 who showed that while people with a higher waist circumference had lower PEH, this was not confirmed for people with a higher BMI. Notably, the populations of these studies were hypertensive, middle-aged and elderly, where an increased BMI is most likely due to the accumulation of body fat, whereas in the present study, the volunteers were practicing exercises that led to increased muscle mass. Therefore, we cannot emphatically state that the BMI difference between the users and controls was an influential factor in the magnitude of the PEH.
Once this new finding is demonstrated, it is relevant hereafter to understand which mechanisms would impair AAS users from obtaining a PEH response to an aerobic exercise session. A higher heart rate at rest and after exercise appears to be an indicator of greater sympathetic nervous system activation, increased plasma catecholamines or even a decreased resting parasympathetic activity, which in fact has been previously demonstrated in users of AAS.23 A more significant cardiac output stimulated by a higher post-exercise heart rate may also explain the smaller PEH. Reduced cardiac output and sympathetic nerve activity have been considered important mechanisms of PEH.24 The lower plasma volume caused by exercise is also a mechanism suggested to affect PEH.25 It is also known that high AAS doses may lead to an impaired hemodynamic and metabolic response26,27 as well as endothelial dysfunction.28 Interestingly, an aerobic exercise session promotes the opposite of these actions, so that reduced sympathetic nervous activity, increased parasympathetic tone29 and enhanced endothelial function12 are among the mechanisms proposed to explain PEH. Therefore, each of these mechanisms involved in PEH must be investigated in future studies.
Data of this study must be considered based on the limitations that may be present. The study showed the inhibition of PEH, which is an important clinical effect of exercise for hypertensive patients. However, the sample studied was not hypertensive, and additional studies need to be conducted with AAS users presenting blood pressure values at hypertensive levels. The AAS group had different numbers of cycles in their lives, as well as different periods between each cycle. When the study was conducted, they used different substances; therefore, it is not possible to determine which specific substance is more or less responsible for the impairment of the post-exercise hypotension response. Similarly, it is not possible to determine whether the frequency of cycles is an influencing factor in the post-exercise blood pressure response. Such questions must be addressed in further studies. It is suggested that measurements of autonomic nervous activity and endothelial function should be applied in further investigations, considering the existence of tools that allow further studies in humans.
ConclusionThis study demonstrated an incapability of AAS users to obtain a reduction in blood pressure after an aerobic exercise session, indicating a new side effect of AAS use.
Conflicts of interestThe authors declare no conflicts of interest.