To describe the main characteristics of low back pain randomized controlled trials on the Physiotherapy Evidence Database, and to rank the journals where these trials were published according to their Impact Factor.
MethodsThis is a cross sectional study based on a collection of randomized controlled trials. A random sample of 200 low back pain trials published between 2010 and 2015 were selected from Physiotherapy Evidence Database in February 2016. We collected the following main characteristics of trials: 2015 journal Impact Factor; if the paper was published as open access; CONSORT recommendations endorsement by the journal; methodological quality and statistical reporting measured by the 0–10 items Physiotherapy Evidence Database scale. Data was analyzed descriptively.
ResultsTrials were published in journals with a mean Impact Factor of 2.5 (SD 2.5), from which 55.5% endorsed the CONSORT recommendations. The methodological quality was moderate with 5.8 points (SD 1.6). The top 3 journals according to Impact Factor were: (1) British Medical Journal; (2) Annals of Internal Medicine; and (3) BMC Medicine. Only 6 out of 97 journals publishing low back pain trials combined the following factors: journal Impact Factor higher than 2.0, mean trial methodological quality higher than 6.0 points, endorse CONSORT recommendations and offering papers as open access.
ConclusionClinicians interested in low back pain trials must look for a wide variety of healthcare journals. A substantial number of low back pain randomized controlled trials did not follow adequate reporting and methodological recommendations.
Low back pain is the leading cause of years lived with disability since 1990,1 with a high prevalence and costs worldwide.2–5 Low back pain affects not only high income countries, but also high middle income and middle socio-demographic index countries.1 In order to measure treatment effectiveness for this condition, the best evidence is provided by randomized controlled trials or systematic reviews of randomized controlled trials.6,7
The usual treatment for low back pain patients is consisted of nonpharmacologic therapies, education, reassurance and analgesic medication.8 Nonpharmacologic therapies have shown effectiveness on mind-body interventions, such as exercise, psychological therapies, multidisciplinary rehabilitation, spinal manipulation, massage and acupuncture.9 Such interventions reinforce the essential role carried by physical therapists in the long-term management of this condition.10
In order to implement treatments for low back pain, randomized controlled trials should follow adequate reporting and methodological guidelines.11 The methodological quality of physical therapy randomized controlled trials varies across different subdisciplines.12 The methodological quality of trials in musculoskeletal physical therapy has been associated with endorsement of reporting recommendations (i.e. trials/journals that formally ask authors to report their trials using the Consolidated Standards of Reporting Trials, CONSORT13), articles published recently and trials published in English.14 On the other hand, the methodological quality is not necessarily associated with journal Impact Factor.15
The Physiotherapy Evidence Database (PEDro; www.pedro.org.au) is an open access database that indexes clinical trials, systematic reviews and guidelines in physical therapy.16 PEDro is one of the four most comprehensive healthcare databases, including CENTRAL, PubMed and EMBASE, but only PEDro is focused only in physical therapy interventions.17,18 Each clinical trial indexed on PEDro goes through a methodological quality and statistical reporting assessment measured by the PEDro scale,19–22 which generates a score that ranges from 0 (low methodological quality) to 10 (high methodological quality). The items from the PEDro scale are: (1) eligibility criteria and source (not included on the total score); (2) random allocation; (3) concealed allocation; (4) baseline comparability; (5) blinding of subjects; (6) blinding of therapists; (7) blinding of assessors; (8) adequate follow-up; (9) intention-to-treat analysis; (10) between-group comparisons; (11) point estimates and variability.
Clinicians treating low back pain patients should be aware about what would be the core journals that publish randomized controlled trials in physical therapy for low back pain. In addition, it is important to understand the strengths and weaknesses of those trials.11 Therefore, the primary objective of this study was to describe the main characteristics of low back pain randomized controlled trials on PEDro, and to rank the journals where these trials were published according to their Impact Factor. Our secondary objective was to analyze the correlation between trials methodological quality and journals Impact Factor.
MethodsStudy selectionWe searched for all low back pain randomized controlled trials, published between 2010 and 2015, and indexed on PEDro database on February 1st 2016. Then we randomly selected a sample of approximately 40% of all eligible trials, using the random number function in Excel. The eligibility criteria were: full-published articles; written in English, Spanish or Portuguese; and published between 2010 and 2015. The languages were restricted to English, Spanish and Portuguese, as they are languages spoken by the authors of this manuscript and are within the most published languages on PEDro.14,23 Trials that were still in the recruitment stages, protocols, duplicates and those involving any topic other than low back pain were excluded. We used the advanced search strategy on PEDro, as follows: “clinical trial” for method; “lumbar spine, sacroiliac joint or pelvis” for body part; “pain” for problem; and 2010–2015 for year of publication.
Data extractionOne independent author extracted data in an Excel spreadsheet previously formatted to describe the trials’ characteristics. Data extracted from the trials were: (1) continent where trial was conducted; (2) language of publication, categorized into English or non-English; (3) methodological quality, which was measured by total PEDro score and downloaded from PEDro database (0–10 points with higher values indicating better methodological quality); (4) number of years since publication, calculated by subtracting the year of publication from 2015; (5) number of citations normalized by the number of years since publication (extracted from the Web of Science Clarivate Analytics); (6) total sample size (i.e., number of participants randomized); (7) primary outcomes, which were collected at most two outcomes. For articles that did not specify the primary outcome, but had two outcomes mentioned only, we considered both as primary. For articles that did not specify the primary outcome but had more than two outcomes, we considered “pain” and “disability” as primary.24 In the cases which the article did not have any of these two outcomes, we collected the first two mentioned by the authors. All primary outcomes were categorized into: pain, disability, function, quality of life or other; (8) interventions, which included any type of physical therapy intervention. Each intervention was categorized according to the intervention codes used by PEDro25 (i.e., Acupuncture; Behavior modification; Education; Electrotherapy, heat, cold; Fitness training; Health promotion; Hydrotherapy, balneotherapy; Neurodevelopmental therapy, neurofacilitation; Ortheses, taping, splinting; Respiratory therapy; Skill training; Strength training; Stretching, mobilization, manipulation, massage; No appropriate value in this field); (9) comparators, which were categorized into no-treatment control,26 placebo,27 addition of other treatment,28 waiting list,29 minimal intervention,28 usual care30 and/or other intervention.31 We also classified the trial in one of these groups if the authors of the trial classified the comparator group as such.
Data extracted from journals were: (1) 2015 journal Impact Factor (from the Web of Science, InCites Journal Citation Reports – Thomson Reuters’ website Clarivate Analytics), and categorized in three strata (Journals without Impact Factor; Journals with Impact Factor less than 2.0; Journals with Impact Factor more than 2.0); (2) if journal was open access (yes or no), which was obtained from the Directory of Open Access Journals, PubMed Central or the journal's web-site; and (3) if journal endorses the CONSORT recommendations32 (yes or no), which was extracted from the “Instructions to authors” for each of the journals or from the CONSORT's website.13
Main outcome measuresThe main outcome measures were: journal Impact Factor; if the paper was published as open access; CONSORT recommendations endorsement by the journal; and methodological quality measured by total PEDro score.
Data analysisAll trials characteristics were analyzed descriptively. Normality tests were conducted and all data were normally distributed, except for the total sample size, which was reported as median and interquartile range. The correlation between trials methodological quality and journals Impact Factor was calculated using Pearson correlation. We used SPSS software (Version 20.0) for all analyses.
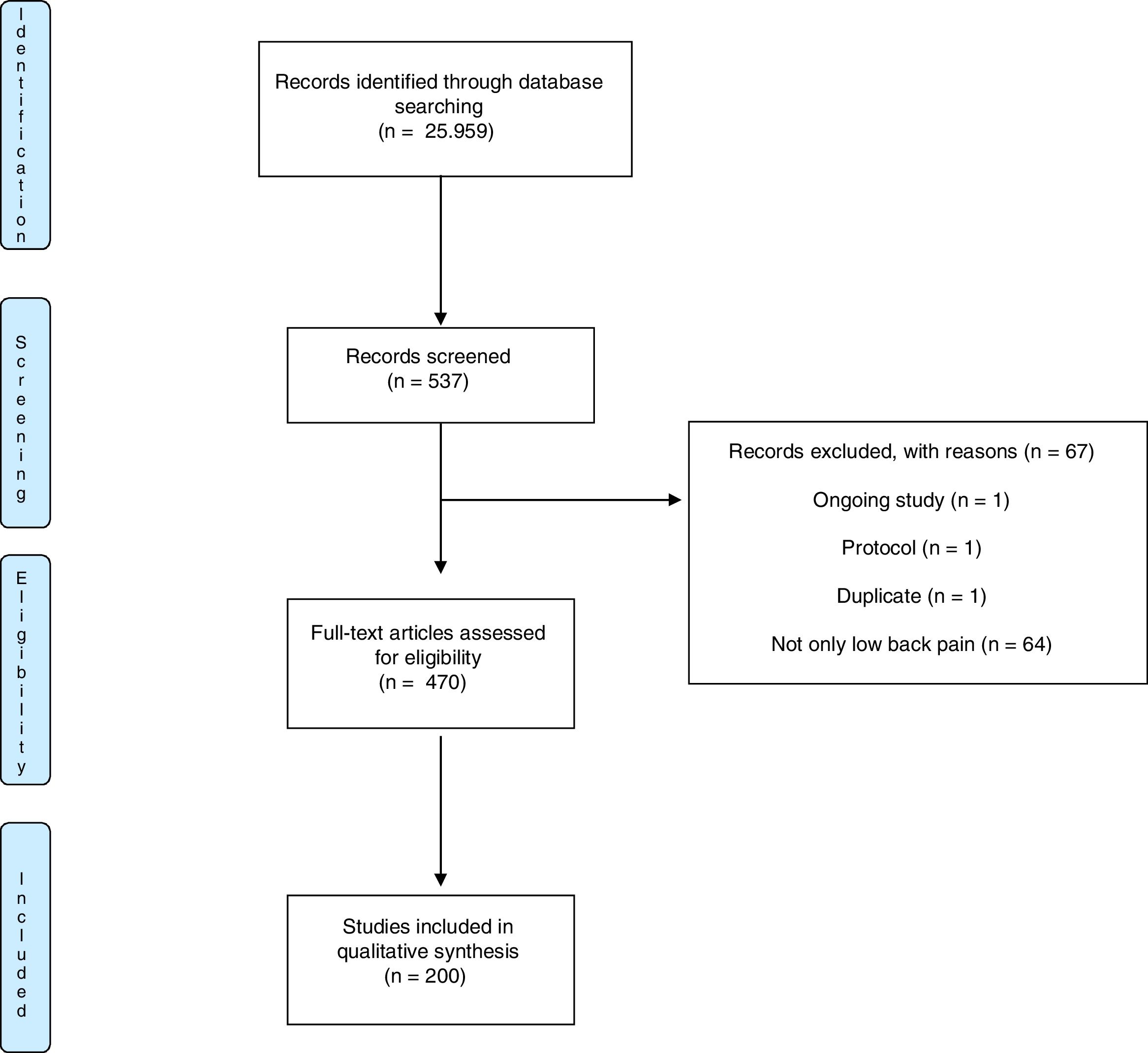
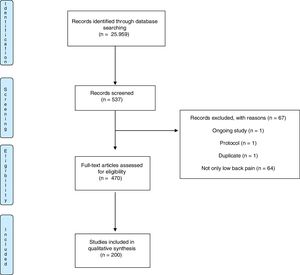
ResultsThe search strategy on PEDro database yielded 25.956 clinical trials. Using the advanced search strategy 537 potentially eligible trials were retrieved. A total of 67 were excluded as these trials were not eligible. From the remaining 470 articles, we randomly selected a sample of 200 trials, represented in the flow chart in Fig. 1.
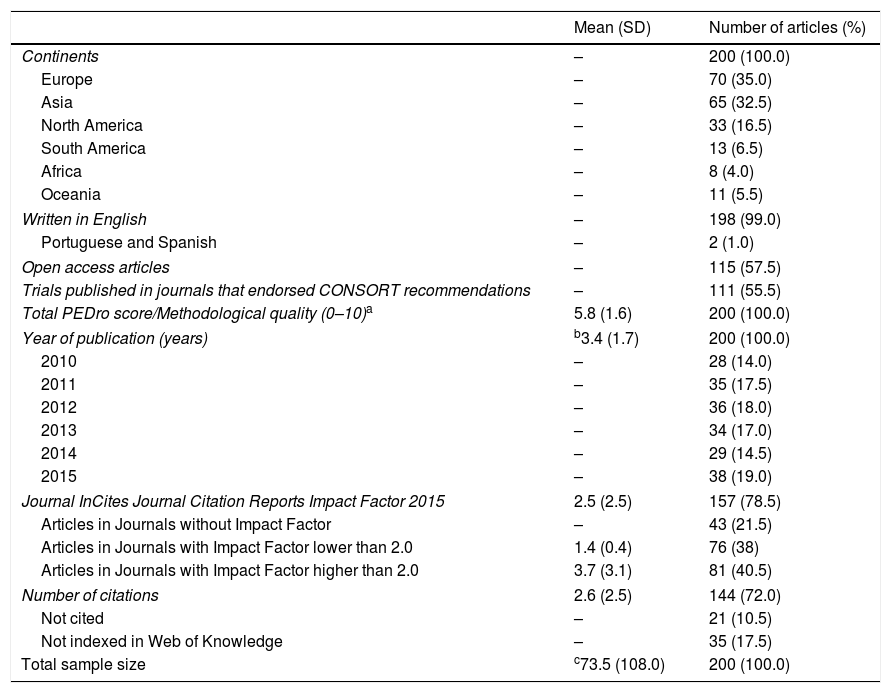
Table 1 presented the descriptive data of the 200 articles. Most of trials were conducted in Europe and Asia, published in English, in open access journals and more than half endorsed CONSORT recommendations.
Descriptive data of the total sample (n=200) with mean (standard deviation) and the frequency (percentage) of articles.
| Mean (SD) | Number of articles (%) | |
|---|---|---|
| Continents | – | 200 (100.0) |
| Europe | – | 70 (35.0) |
| Asia | – | 65 (32.5) |
| North America | – | 33 (16.5) |
| South America | – | 13 (6.5) |
| Africa | – | 8 (4.0) |
| Oceania | – | 11 (5.5) |
| Written in English | – | 198 (99.0) |
| Portuguese and Spanish | – | 2 (1.0) |
| Open access articles | – | 115 (57.5) |
| Trials published in journals that endorsed CONSORT recommendations | – | 111 (55.5) |
| Total PEDro score/Methodological quality (0–10)a | 5.8 (1.6) | 200 (100.0) |
| Year of publication (years) | b3.4 (1.7) | 200 (100.0) |
| 2010 | – | 28 (14.0) |
| 2011 | – | 35 (17.5) |
| 2012 | – | 36 (18.0) |
| 2013 | – | 34 (17.0) |
| 2014 | – | 29 (14.5) |
| 2015 | – | 38 (19.0) |
| Journal InCites Journal Citation Reports Impact Factor 2015 | 2.5 (2.5) | 157 (78.5) |
| Articles in Journals without Impact Factor | – | 43 (21.5) |
| Articles in Journals with Impact Factor lower than 2.0 | 1.4 (0.4) | 76 (38) |
| Articles in Journals with Impact Factor higher than 2.0 | 3.7 (3.1) | 81 (40.5) |
| Number of citations | 2.6 (2.5) | 144 (72.0) |
| Not cited | – | 21 (10.5) |
| Not indexed in Web of Knowledge | – | 35 (17.5) |
| Total sample size | c73.5 (108.0) | 200 (100.0) |
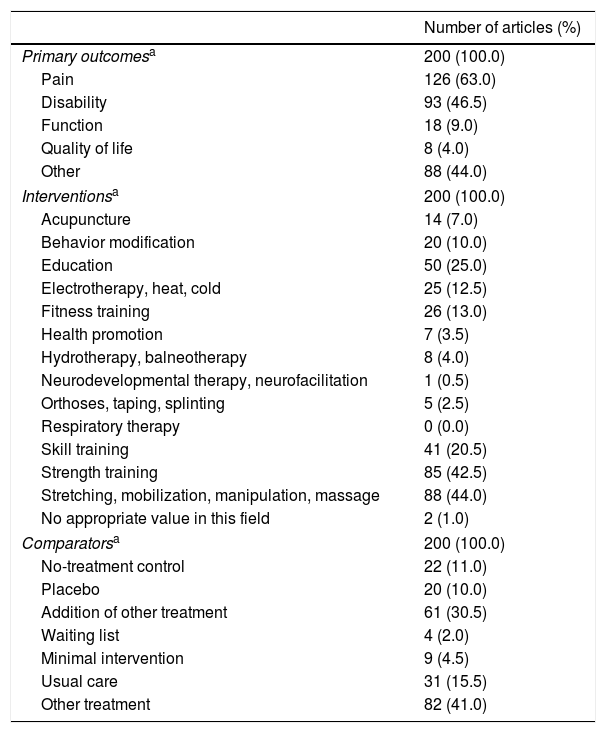
Table 2 presented the frequency and percentage of the primary outcomes, interventions and comparators. Most of trials evaluated pain and disability as primary outcomes; strength training, stretching, mobilization, manipulation and/or massage as interventions; and were mainly compared with other treatment or the addition of other treatment.
Frequency (percentage) of the primary outcomes, interventions and comparators presented in the total sample (n=200).
| Number of articles (%) | |
|---|---|
| Primary outcomesa | 200 (100.0) |
| Pain | 126 (63.0) |
| Disability | 93 (46.5) |
| Function | 18 (9.0) |
| Quality of life | 8 (4.0) |
| Other | 88 (44.0) |
| Interventionsa | 200 (100.0) |
| Acupuncture | 14 (7.0) |
| Behavior modification | 20 (10.0) |
| Education | 50 (25.0) |
| Electrotherapy, heat, cold | 25 (12.5) |
| Fitness training | 26 (13.0) |
| Health promotion | 7 (3.5) |
| Hydrotherapy, balneotherapy | 8 (4.0) |
| Neurodevelopmental therapy, neurofacilitation | 1 (0.5) |
| Orthoses, taping, splinting | 5 (2.5) |
| Respiratory therapy | 0 (0.0) |
| Skill training | 41 (20.5) |
| Strength training | 85 (42.5) |
| Stretching, mobilization, manipulation, massage | 88 (44.0) |
| No appropriate value in this field | 2 (1.0) |
| Comparatorsa | 200 (100.0) |
| No-treatment control | 22 (11.0) |
| Placebo | 20 (10.0) |
| Addition of other treatment | 61 (30.5) |
| Waiting list | 4 (2.0) |
| Minimal intervention | 9 (4.5) |
| Usual care | 31 (15.5) |
| Other treatment | 82 (41.0) |
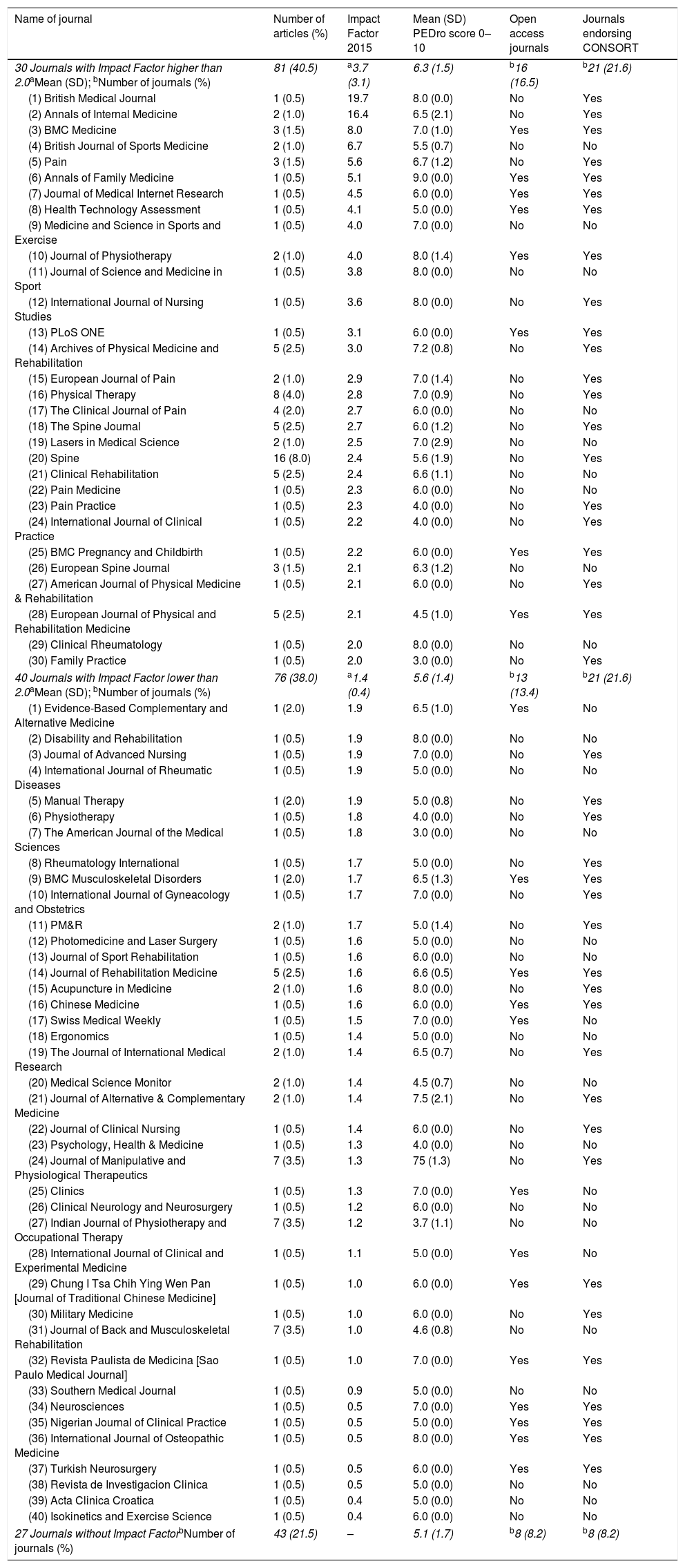
The 200 trials were published in 97 different journals. We organized the journals into: Impact Factor higher than 2.0 (30 journals with 81 articles); Impact Factor lower than 2.0 (40 journals with 76 articles); and journals without Impact Factor (27 journals with 43 articles) (Table 3). The top 3 journals were: (1) British Medical Journal; (2) Annals of Internal Medicine; and (3) BMC Medicine. As our results showed, the higher the journal Impact Factor, the higher the methodological quality of the trials, and apparently is also higher the likelihood of the journals to endorse CONSORT recommendations and offer open access (Table 3). Combining those four characteristics together (Impact Factor higher than 2.0, trial methodological quality higher than 6.0, endorse CONSORT recommendations and open access journal), the top 3 journals were: (1) BMC Medicine; (2) Annals of Family Medicine; and (3) Journal of Medical Internet Research. Interestingly, only three other journals from our sample also presented those four characteristics, which were the Journal of Physiotherapy, PLoS ONE and BMC Pregnancy and Childbirth.
Descriptive data of 200 trials published in 97 journals. Journals categorized into Impact Factor higher and lower than 2.0, and journals without Impact Factor. Ranking of journals is presented from higher to lower Impact factors (70 journals).
| Name of journal | Number of articles (%) | Impact Factor 2015 | Mean (SD) PEDro score 0–10 | Open access journals | Journals endorsing CONSORT |
|---|---|---|---|---|---|
| 30 Journals with Impact Factor higher than 2.0aMean (SD); bNumber of journals (%) | 81 (40.5) | a3.7 (3.1) | 6.3 (1.5) | b16 (16.5) | b21 (21.6) |
| (1) British Medical Journal | 1 (0.5) | 19.7 | 8.0 (0.0) | No | Yes |
| (2) Annals of Internal Medicine | 2 (1.0) | 16.4 | 6.5 (2.1) | No | Yes |
| (3) BMC Medicine | 3 (1.5) | 8.0 | 7.0 (1.0) | Yes | Yes |
| (4) British Journal of Sports Medicine | 2 (1.0) | 6.7 | 5.5 (0.7) | No | No |
| (5) Pain | 3 (1.5) | 5.6 | 6.7 (1.2) | No | Yes |
| (6) Annals of Family Medicine | 1 (0.5) | 5.1 | 9.0 (0.0) | Yes | Yes |
| (7) Journal of Medical Internet Research | 1 (0.5) | 4.5 | 6.0 (0.0) | Yes | Yes |
| (8) Health Technology Assessment | 1 (0.5) | 4.1 | 5.0 (0.0) | Yes | Yes |
| (9) Medicine and Science in Sports and Exercise | 1 (0.5) | 4.0 | 7.0 (0.0) | No | No |
| (10) Journal of Physiotherapy | 2 (1.0) | 4.0 | 8.0 (1.4) | Yes | Yes |
| (11) Journal of Science and Medicine in Sport | 1 (0.5) | 3.8 | 8.0 (0.0) | No | No |
| (12) International Journal of Nursing Studies | 1 (0.5) | 3.6 | 8.0 (0.0) | No | Yes |
| (13) PLoS ONE | 1 (0.5) | 3.1 | 6.0 (0.0) | Yes | Yes |
| (14) Archives of Physical Medicine and Rehabilitation | 5 (2.5) | 3.0 | 7.2 (0.8) | No | Yes |
| (15) European Journal of Pain | 2 (1.0) | 2.9 | 7.0 (1.4) | No | Yes |
| (16) Physical Therapy | 8 (4.0) | 2.8 | 7.0 (0.9) | No | Yes |
| (17) The Clinical Journal of Pain | 4 (2.0) | 2.7 | 6.0 (0.0) | No | No |
| (18) The Spine Journal | 5 (2.5) | 2.7 | 6.0 (1.2) | No | Yes |
| (19) Lasers in Medical Science | 2 (1.0) | 2.5 | 7.0 (2.9) | No | No |
| (20) Spine | 16 (8.0) | 2.4 | 5.6 (1.9) | No | Yes |
| (21) Clinical Rehabilitation | 5 (2.5) | 2.4 | 6.6 (1.1) | No | No |
| (22) Pain Medicine | 1 (0.5) | 2.3 | 6.0 (0.0) | No | No |
| (23) Pain Practice | 1 (0.5) | 2.3 | 4.0 (0.0) | No | Yes |
| (24) International Journal of Clinical Practice | 1 (0.5) | 2.2 | 4.0 (0.0) | No | Yes |
| (25) BMC Pregnancy and Childbirth | 1 (0.5) | 2.2 | 6.0 (0.0) | Yes | Yes |
| (26) European Spine Journal | 3 (1.5) | 2.1 | 6.3 (1.2) | No | No |
| (27) American Journal of Physical Medicine & Rehabilitation | 1 (0.5) | 2.1 | 6.0 (0.0) | No | Yes |
| (28) European Journal of Physical and Rehabilitation Medicine | 5 (2.5) | 2.1 | 4.5 (1.0) | Yes | Yes |
| (29) Clinical Rheumatology | 1 (0.5) | 2.0 | 8.0 (0.0) | No | No |
| (30) Family Practice | 1 (0.5) | 2.0 | 3.0 (0.0) | No | Yes |
| 40 Journals with Impact Factor lower than 2.0aMean (SD); bNumber of journals (%) | 76 (38.0) | a1.4 (0.4) | 5.6 (1.4) | b13 (13.4) | b21 (21.6) |
| (1) Evidence-Based Complementary and Alternative Medicine | 1 (2.0) | 1.9 | 6.5 (1.0) | Yes | No |
| (2) Disability and Rehabilitation | 1 (0.5) | 1.9 | 8.0 (0.0) | No | No |
| (3) Journal of Advanced Nursing | 1 (0.5) | 1.9 | 7.0 (0.0) | No | Yes |
| (4) International Journal of Rheumatic Diseases | 1 (0.5) | 1.9 | 5.0 (0.0) | No | No |
| (5) Manual Therapy | 1 (2.0) | 1.9 | 5.0 (0.8) | No | Yes |
| (6) Physiotherapy | 1 (0.5) | 1.8 | 4.0 (0.0) | No | Yes |
| (7) The American Journal of the Medical Sciences | 1 (0.5) | 1.8 | 3.0 (0.0) | No | No |
| (8) Rheumatology International | 1 (0.5) | 1.7 | 5.0 (0.0) | No | Yes |
| (9) BMC Musculoskeletal Disorders | 1 (2.0) | 1.7 | 6.5 (1.3) | Yes | Yes |
| (10) International Journal of Gyneacology and Obstetrics | 1 (0.5) | 1.7 | 7.0 (0.0) | No | Yes |
| (11) PM&R | 2 (1.0) | 1.7 | 5.0 (1.4) | No | Yes |
| (12) Photomedicine and Laser Surgery | 1 (0.5) | 1.6 | 5.0 (0.0) | No | No |
| (13) Journal of Sport Rehabilitation | 1 (0.5) | 1.6 | 6.0 (0.0) | No | No |
| (14) Journal of Rehabilitation Medicine | 5 (2.5) | 1.6 | 6.6 (0.5) | Yes | Yes |
| (15) Acupuncture in Medicine | 2 (1.0) | 1.6 | 8.0 (0.0) | No | Yes |
| (16) Chinese Medicine | 1 (0.5) | 1.6 | 6.0 (0.0) | Yes | Yes |
| (17) Swiss Medical Weekly | 1 (0.5) | 1.5 | 7.0 (0.0) | Yes | No |
| (18) Ergonomics | 1 (0.5) | 1.4 | 5.0 (0.0) | No | No |
| (19) The Journal of International Medical Research | 2 (1.0) | 1.4 | 6.5 (0.7) | No | Yes |
| (20) Medical Science Monitor | 2 (1.0) | 1.4 | 4.5 (0.7) | No | No |
| (21) Journal of Alternative & Complementary Medicine | 2 (1.0) | 1.4 | 7.5 (2.1) | No | Yes |
| (22) Journal of Clinical Nursing | 1 (0.5) | 1.4 | 6.0 (0.0) | No | Yes |
| (23) Psychology, Health & Medicine | 1 (0.5) | 1.3 | 4.0 (0.0) | No | No |
| (24) Journal of Manipulative and Physiological Therapeutics | 7 (3.5) | 1.3 | 75 (1.3) | No | Yes |
| (25) Clinics | 1 (0.5) | 1.3 | 7.0 (0.0) | Yes | No |
| (26) Clinical Neurology and Neurosurgery | 1 (0.5) | 1.2 | 6.0 (0.0) | No | No |
| (27) Indian Journal of Physiotherapy and Occupational Therapy | 7 (3.5) | 1.2 | 3.7 (1.1) | No | No |
| (28) International Journal of Clinical and Experimental Medicine | 1 (0.5) | 1.1 | 5.0 (0.0) | Yes | No |
| (29) Chung I Tsa Chih Ying Wen Pan [Journal of Traditional Chinese Medicine] | 1 (0.5) | 1.0 | 6.0 (0.0) | Yes | Yes |
| (30) Military Medicine | 1 (0.5) | 1.0 | 6.0 (0.0) | No | Yes |
| (31) Journal of Back and Musculoskeletal Rehabilitation | 7 (3.5) | 1.0 | 4.6 (0.8) | No | No |
| (32) Revista Paulista de Medicina [Sao Paulo Medical Journal] | 1 (0.5) | 1.0 | 7.0 (0.0) | Yes | Yes |
| (33) Southern Medical Journal | 1 (0.5) | 0.9 | 5.0 (0.0) | No | No |
| (34) Neurosciences | 1 (0.5) | 0.5 | 7.0 (0.0) | Yes | Yes |
| (35) Nigerian Journal of Clinical Practice | 1 (0.5) | 0.5 | 5.0 (0.0) | Yes | Yes |
| (36) International Journal of Osteopathic Medicine | 1 (0.5) | 0.5 | 8.0 (0.0) | Yes | Yes |
| (37) Turkish Neurosurgery | 1 (0.5) | 0.5 | 6.0 (0.0) | Yes | Yes |
| (38) Revista de Investigacion Clinica | 1 (0.5) | 0.5 | 5.0 (0.0) | No | No |
| (39) Acta Clinica Croatica | 1 (0.5) | 0.4 | 5.0 (0.0) | No | No |
| (40) Isokinetics and Exercise Science | 1 (0.5) | 0.4 | 6.0 (0.0) | No | No |
| 27 Journals without Impact FactorbNumber of journals (%) | 43 (21.5) | – | 5.1 (1.7) | b8 (8.2) | b8 (8.2) |
Trials methodological quality and statistical reporting measured by the PEDro scale was weakly correlated with journals Impact Factor (r=0.23; p=0.004).
DiscussionMain characteristics of low back pain trialsLow back pain randomized controlled trials have been published in a variety of healthcare journals, with Impact Factors ranging from 0.4 to 19.7, and even in some journals that were not even indexed in the Web of Science. Most of these trials had moderate methodological quality (with a PEDro mean score of 5.8 points out of 10.0) and were published in open access journals that endorsed CONSORT recommendations. Similar to a previous study in musculoskeletal physical therapy,14 our results indicate a positive, but weak correlation between methodological quality and Impact Factor. This means that high quality low back pain trials are not necessarily published in journals with high Impact Factor.
Low back pain trials characteristics and the literatureThe sample size showed a median of 73.5 participants (IQR 109, minimum 10–maximum 4.325), which shows that some studies analyzed a small group of participants, which probably did not reach the statistical power of detecting a clinical important difference among the intervention and control groups.33
The primary outcomes most evaluated by the trials were pain and disability, which agrees with the core outcome sets found in panel consensus for low back pain, together with quality of life.34 The instruments mostly recommended for physical functioning are the Oswestry Disability Index and 24-item Roland-Morris Disability Questionnaire, and the Numerical Pain Rating Scale.34 This shows that most of back pain researchers are aligned with these recommendations.
The most frequent interventions analyzed in our sample were exercises (strength training and stretching), manual therapy and education, which are the main recommended interventions for low back pain according to the National Institute for Health and Care Excellence (NICE) guidelines,35 and also to a recent low back pain series published by a panel of experts in the field.2–4 Maybe authors should investigate further the effectiveness of interventions that were poorly or not investigated in these trials for low back pain and which do not present much evidence according to previously published guidelines,2–4,35 such as hydrotherapy and neurodevelopment therapy, for example.
Strengths and limitationsA particular strength of our study is that we presented 30 possible options of high Impact Factor journals that published low back pain trials, from which six journals are highly recommended for reading and referred to when taking a clinical decision. Also, we are confident that we have a representative sample of low back pain trials published on PEDro in 2010–2015, once we selected 40% of the total ‘population’ of eligible trials. However, our main limitation is that some journals might have been missed and we could not generalize our results for all low back pain trials, including trials involving surgery or drug trials.
Clinical and methodological implicationsThe analyzed trials comparing interventions for low back pain presented moderate methodological quality. Readers should be very cautious on taking clinical decisions based on poor methodological quality trials, which may increase the existing gap between evidence and practice.2–4 The research efforts and global initiatives to treat this public health problem need also to focus on improving reporting and methodological quality for future studies. Although 55.5% of journals already endorse CONSORT recommendations, we suggest that the EQUATOR recommendations (Enhancing the QUAlity and Transparency Of health Research; https://www.equator-network.org/) become mandatory requirements for publication, as well as following the items evaluated in methodological guidelines (e.g. Cochrane Risk of Bias tool or the PEDro scale36). Journal editors and peer reviewers should strictly follow those requirements in order to improve reporting and methodological quality of randomized controlled trials. Therefore, actions of better reporting and conducting the studies following adequate methodological guidelines will better address authors and journals editors/reviewers to write and publish more trustworthy studies.
ConclusionLow back pain randomized controlled trials in the field of physical therapy presented moderate methodological quality. Our findings also show that trial quality was weakly correlated with Impact Factor. Clinicians interested in low back pain trials may look for a wide variety of healthcare journals. A substantial number of low back pain randomized controlled trials did not follow adequate reporting and methodological recommendations.
FundingThis work was supported by a scholarship from the Sao Paulo Research Foundation (FAPESP), under the process number of 2015/16953-2.
Conflicts of interestThe authors declare no financial competing interest. Furthermore, seven out of the 200 articles analyzed involved authors from our research groups or collaborators.