Whole body vibration has been used alone or combined with other interventions in rehabilitation of children and adolescents with disabilities; however, there is limited evidence to support this approach.
ObjectivesTo review the strength, quality, and conclusiveness of evidence supporting the use of whole body vibration in children and adolescents with disabilities.
MethodsElectronic database search included Medline, AMED, Embase, Cochrane, SportDiscus, CINAHL and PEDro from the inception to June 2018. Studies investigating the effects of whole body vibration, alone or combined with other interventions, compared to minimal intervention or other interventions were included. The outcomes measured were: body structure and function (lean body mass, bone mineral density, knee muscle strength and overall stability) and activity and participation (gait speed, walking distance, gross motor function, self-care and mobility).
ResultsFifteen randomized trials involving 403 participants were included. Methodological quality of eligible trials was moderate (mean of 5.5 points on the 10-point PEDro scale). Overall, whole body vibration was no better than minimal intervention. In all comparisons where additional effect of whole body vibration was better than other interventions, the effect size ranged from low to high in the trials, but ranged from very-low to low quality at short and medium-term follow-up. Sensitivity analysis for health condition and low-quality studies showed impact on trunk bone mineral density of additional effect of whole body vibration at medium-term compared to other interventions.
ConclusionThe low to very-low quality of evidence suggests caution in recommending the use of this approach. New studies could change the findings of this review. PROSPERO registration: CRD42017060704.
Children and adolescents with disabilities are characterized as having impairments in body structure and function, limitation of activity and social restriction in accordance to International Classification of Functioning, Disability and Health (ICF).1–6 Disability is a generic term and may involve neurological, musculoskeletal, metabolic and burned-related conditions in the childhood and adolescence.7 Considering different levels of disability, these individuals can present low bone mineral density, altered body composition, decreased levels of muscle strength, instability, reduced walking capacity, reduced gross motor function, and activities of daily living performance.8–10 Furthermore, children and adolescents with disabling conditions have reduced levels of habitual activity compared with their typically developing peers, as recommended by guidelines.11–14 These individuals are limited to engage in some physical activity programs due many barriers, such as sedentary lifestyles, motor deficits, social and recreation opportunities, or low motivation to be physically active.15 New approaches have been used to minimize deficiencies and activity limitations of these population,16 such as the use of whole body vibration (WBV).
WBV has become a very popular treatment for disabilities in children,17 adolescents18 and adults19 over the last decade. WBV is a type of training that uses high-frequency mechanical stimuli, which is generated by a vibrating platform and transmitted through body to load bone and stimulate sensory receptors.12 Previous studies using WBV showed improvements on muscle strength, lean mass, bone mass, stability, gait capacity and gross motor performance.20–23 Although WBV is used in clinical practice by physical therapists worldwide, its effectiveness in children and adolescent with disabilities is still unclear due to limited methodological quality of previous studies.24–31
A previous systematic review investigated effects of WBV on health-related physical fitness in children and adolescents with disabilities.32,33 Authors reported that WBV may be effective on disabled children and adolescents’ health; however, they included low quality studies (i.e. not randomized controlled trials), did not assess risk of bias of the included trials, and meta-analysis was not conducted.31 Moreover, the role of WBV in physical rehabilitation in children and adolescent with disabilities is uncertain because training can be used alone or in conjunction with other interventions,14 once there is the possibility that the scientific evidence of one intervention differs from the effect of the other and even from the combination of both.34 Therefore, in an attempt to address these gaps, the research question for this systematic review of randomized controlled trials was: is WBV alone or combined with other interventions more effective than minimal or other interventions in children and adolescent with disabilities?
MethodsThis study was a systematic review with meta-analysis. Review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement,35 and its protocol was prospectively registered at International prospective register of systematic reviews (PROSPERO)36 (CRD42017060704).
Identification and selection of the studiesSearch strategies were conducted on Medline, AMED, Embase, Cochrane, SportDiscus, CINAHL and PEDro in September 2016 and updated in June 2018, without language or date restrictions. Descriptors used in our search strategy were related to “randomized controlled trials”, “children”, “adolescent” and “whole body vibration”. We also hand searched identified systematic reviews in the field.
Screening of identified titles and abstracts, and assessment of potential full-texts using our eligibility criteria was conducted by two independent reviewers (ACRC and HRL), with discrepancies resolved by a third reviewer (VCO).
Assessment of characteristic of studiesQualityThe methodological quality of the included trials was assessed by extracting the PEDro score from the Physiotherapy Evidence Database (www.pedro.org.au).37 If the trial was not included in the database the score was assessed by two independent reviewers (ACRC and HRL) using the 0–10 PEDro scale. A third reviewer (VCO) resolved discrepancies. The PEDro scale assesses 10 criteria and final score ranges from 0 to 10, with greater scores meaning greater methodological quality.37 Reviewers were trained a priori.
ParticipantsIn the current review, disability was defined as a complex phenomenon which covers impairments, activity limitations and participation restrictions according to definition of World Health Organization (WHO).6 Studies were eligible if they included randomized controlled trials that investigated the effects of WBV in children and adolescent with disabilities, such as neurological, musculoskeletal, metabolic and burn-related conditions.
InterventionThe experimental intervention was WBV which was defined as any mechanical stimulus characterized by an oscillatory motion generated by vibrating devices using two different systems: (a) reciprocating vertical displacements on the left and right side of a fulcrum; (b) the whole plate oscillating uniformly up and down.32,33 Trials were considered for inclusion if they compared WBV to minimal intervention (i.e. no intervention, waiting list, placebo and sham) or additional effect of WBV compared to other interventions.
OutcomesWe were interested on primary (related to body and structure and function: lean body mass, bone mineral density, muscle strength and overall stability) and secondary outcomes (related to activity and participation: gait speed/walking distance, gross motor function, self-care and mobility), according to Classification of Functioning and Disability and Health.6
Data analysisData were extracted by two independent reviewers (ACRC and HRL), with a third reviewer (VCO) resolving potential discrepancies. Extracted data included characteristics of included studies (i.e. participants, interventions, outcomes, and type of platform), and outcome measures (i.e. means, standard deviations, and sample sizes). Outcome measures were extracted for short-, medium-, and long-term effects. Short-term effect was considered up to twelve weeks after baseline, medium-term was considered over twelve weeks but less than twelve months after baseline, and long-term effect was considered at least twelve months after baseline. When more than one timepoint was available for short-term effect, we considered the one closer to the end of the intervention. When outcome measures were not provided in the study, data were imputed following the Cochrane recommendations.38 For bone mineral density, assessed regions varied across studies, so we arbitrarily decided to extract data, firstly, for leg or trunk. Then, data for other regions were considered in studies assessing other regions (i.e. tibia, femur, lumbar, and total bone mineral density).
Outcome data were pooled in meta-analysis using different health conditions and results were presented as standardized mean difference (SMD) with 95% confidence intervals (CIs). Between-trial heterogeneity was assessed using I2, and random-effects model was used considering clinical heterogeneity and when appropriate (i.e. I2≥50%).38 To judge the clinical relevance effects of changes provided by WBV, effect sizes were assessed using Cohen's benchmarks: d<0.2 for small; d>0.5 for medium; and >0.8 for large effects.39 There are systematic reviews that pooled different disabilities40 and it is known that this can impact on outcomes.41 Thus, we conducted sensitivity analysis to investigate impact of methodological quality and health condition on estimated effects.42 Software Comprehensive Meta-analysis 2.2.04 (Biostat, Englewood, NJ) was used for all analysis.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used to summarize the overall quality of the current evidence.43 The four levels of the GRADE system range from high-quality to very low-quality evidence.43 Quality of evidence scoring for each outcome using GRADE started at high-quality evidence, which was downgraded by one point if one of the following criteria was present: (i) poor methodological quality for average PEDro score less than five out of 10; (ii) inconsistency of estimates among trials for I2≥50%, heterogeneity or absence of pooling; (iii) indirectness when participants were selected by no reliable methods or when their inclusion criteria in any of the analyzed trials was not clear; (iv) imprecision for samples <300 participants for each outcome44; and (v) publication bias or when its analysis was not possible due to small number of trials (i.e. n<10 trials).45 Two independent reviewers (HRL and ACRC) assessed current evidence and a third reviewer (VCO) resolved potential discrepancies.
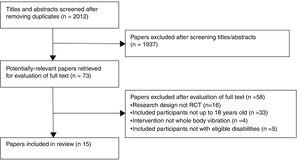
ResultsFlow of the studies through the reviewSearches identified 2012 references, 73 potential full-texts were assessed for our eligibility criteria, and 15 original studies were included in this review (Fig. 1).
Characteristics of the included studiesCharacteristics of included trials are in Table 1. All 15 included randomized controlled trials were published in English between 2004 and 2017.
Descriptive data of the included trials (n=15).
| Trial | Source | Participants | Intervention | Outcome measures | Type of platform |
|---|---|---|---|---|---|
| WBV compared to minimal intervention | |||||
| Neurological conditions | |||||
| González-Aguero et al.50 2013 | Adolescents with Down syndrome, boys and girls, recruited from different schools in Aragon (Spain). | n=30Experimental: n=16Age (yr)mean(SD): 15.3 (2.6)Control: n=14Age (yr)mean (SD): 15.8 (3.0) | Experimental: WBV25–30Hz, 2mm, squatting position (15–20min, 3/wk×20 wks)Control: no intervention | Lean and fat body mass=DXAMedium term effects=20 wks | Power Plate® Pro5=Vertical vibration platform |
| Matute-Llorente et al.55 2015 | Adolescents with Down syndrome, boys and girls, recruited from three schools and institutions of Aragón (Spain). | n=30Experimental: n=16Age (yr)mean (SD): 15.2 (2.5)Control: n=14Age (yr)mean (SD): 15.5 (3.0) | Experimental: WBV, 25–30Hz, 2mm, squatting position, bent knees at 90° (5–10min, 3/wk×20 wks)Control: normal daily routine | BMD and BMC=DXABone strength indexes, bone morphometry, vBMD, and trabecular bone microarchitecture of tibia and radius=Stratec XCT-2000L pQCT scannerMedium term effects=20 wks | Power Plate® Pro5=synchronous vibration platform |
| Metabolic conditions | |||||
| Erceg et al.51 2015 | Overweight, Latino boys, recruited from Los Angeles area. | n=32Experimental: n=16Age (yr)mean (SD): 9 (1)Control: n=16Age (yr)mean (SD): 9 (1) | Experimental: WBV, 30–40Hz, low and high amplitude, squat, wide-stance squat, calf raise, lunge and modified push-up (3–20min, 3/wk×10 wk)Control: normal daily routine for the 10 wk trial period | BMD and BMC=DXABone biomarkers=osteocalcin and collagen type I C-telopeptideShort term effects=10 wks | NEXTgeneration Power Plate®=vibration platforms simultaneously oscillates in the vertical, anterior–posterior and mediolateral planes |
| WBV compared to other intervention | |||||
| Neurological conditions | |||||
| Eid20 2015 | Children with Down syndrome, boys and girls, recruited from outpatient clinic of Faculty of Physical Therapy, Cairo, Egypt. | n=30Experimental: n=15Age (yr)mean (SD): 8.9 (0.7)Control: n=15Age (yr)mean (SD): 9.3 (0.8) | Experimental: WBV, 25–30Hz, 2mm, standing with knees semi-squats (30°) (5–10min, 3/wk×24 wks)+Physical Therapy program (1h, 3/wk×24 wks)Control: Physical Therapy program (1h, 3/wk×24 wks) | Overall stability index, mediolateral stability index and anteroposterior stability index=Biodex Stability SystemMuscle strength (knee extension and flexion)=Handheld dynamometerMedium term effects=24 wks | Vibraflex Home Edition II=Side-to-side alternating vertical sinusoidal vibration |
| El-Shamy and Mohamed21 2012 | Children with spastic diplegic cerebral palsy, boys and girls, recruited from outpatient clinic at the Faculty of Physical Therapy, Cairo, Egypt. | n=30Experimental: n=15Age (yr)mean (SD): 11.7 (0.8)Control: n=15Age (yr)mean (SD): 11.6 (1.1) | Experimental: WBV, static standing position with the knees bent, 25Hz, 1.7mm (10min/day, 5/wk×24 wks)+Physical Therapy Exercise Program based on neuro-developmental techniqueControl: Physical Therapy Exercise Program based on neuro-developmental technique | Femur, lumbar spine and total body BMD=DXAMedium term effects=24 wks | Not specified |
| El-Shamy46 2014 | Children with spastic diplegic cerebral palsy, boys and girls, recruited from outpatient clinic at the Faculty of Physical Therapy, Cairo, Egypt. | n=30Experimental: n=15Age (yr)mean (SD): 9.7 (1.2)Control: n=15Age (yr)mean (SD): 9.9 (1.1) | Experimental: WBV, 12–18Hz, 2–4mm, standing with knees flexed (10–45°) (9min, 5/wk×12 wks)+Traditional Physical Therapy Program (1h, 5/wk×12 wks)Control: Traditional Physical Therapy Program (1h, 5/wk×12 wks) | Muscle strength (knee extension)=Isokinetic dynamometerOverall stability index, mediolateral stability index and anteroposterior stability index=Biodex Stability SystemShort term effects=12 wks | Vibraflex Home Edition II=Side-to-side alternating vertical sinusoidal vibration |
| Ibrahim et al.23 2014 | Children with diplegic spastic cerebral palsy, boys and girls, recruited from the outpatient clinic of the College of Physical Therapy, Cairo University. | n=30Experimental: n=15Age (yr)mean (SD): 9.6 (1.4)Control: n=15Age (yr)mean (SD): 9.6 (1.4) | Experimental: WBV, 12–18Hz, 4–6mm, with the knees slightly bent (9min, 3/wk×12 wks)+Traditional Physical Therapy Program (1h, 3/wk×12 wks)Control: Traditional Physical Therapy Program (1h, 3/wk×12 wks) | Knee extensor muscle strength=Handheld dynamometerSpasticity of hip adductors, knee extensors, and ankle plantar flexors=Ashworth scaleWalking distance=6-min walking testBalance in basic mobility maneuvers=TUGGross motor function=GMFM-88Short term effects=12 wks | Power Plate=Side-alternating vibration platform, unsynchronized multidimensional oscillations along the sagittal axis |
| Ko et al.47 2016 | Children with spastic cerebral palsy, boys and girls, Republic of Korea. | n=24Experimental: n=12Age (yr)mean (SD): 9.4 (2.7)Control: n=12Age (yr)mean (SD): 9.5 (2.2) | Experimental: WBV, 20–24Hz, 1–2mm, with their knees flexed at 30° (9min, 2/wk×3weeks)+Traditional Physical Therapy Program (30min, 2/wk×3 wks)Control: Traditional Physical Therapy Program (30min, 2/wk×3 wks) | Overall stability=Tetrax Interactive Balance SystemGait speed=10m walk testJoint Position Sense=Tiltmeter (iPhone application)Gait analysis=OptoGait SystemShort term effects=3 wks | Galileo System vibration unit=Side-to-side alternating vertical sinusoidal vibration |
| Lee and Chon22 2013 | Children with cerebral palsy with spastic diplegia or quadriplegia, boys and girls, recruited from a local special school and pediatric rehabilitation center. | n=30Experimental: n=15Age (yr)mean (SD): 10.0 (2.3)Control: n=15Age (yr)mean (SD): 9.7 (2.6) | Experimental: WBV, 5–25Hz, 2–6mm, squat ranging from 30 to 100 degrees of knee flexion (18min, 3/wk×8 wks)+Traditional Physical Therapy Program (30min, 3/wk×8 wks)Control: Conventional physical therapy program (30min, 3/wk×8 weeks) | Gait speed, stride length, cycle time and sagittal kinematics=Three-dimensional gait analyses (Qualisys)Leg muscle thickness of the leg muscles=ultrasound imageShort term effects=8 wks | Galileo System=Side-to-side alternating vertical sinusoidal vibration |
| Ruck et al.48 2010 | Children with cerebral palsy, boys and girls, recruited of a primary school for children with special needs (Ecole Victor-Doré, Montreal). | n=20Experimental: n=10Age (yr)mean: 8.3Control: 10Age (yr)mean: 8.1 | Experimental: WBV, 12–18Hz, 2–6mm, knees and hips between 10° and 45° (9min, 5/wk×24 wks)+School physical therapy programControl: School physical therapy program | Gross motor function=GMFMGait speed=10m walk testBMD of the lumbar spine and femur=DXAMedium term effects=24 wks | Vibraflex Home Edition II®=Side-to-side alternating vertical sinusoidal vibration |
| Stark et al.42 2016 | Toddlers with cerebral palsy, boys and girls, recruited through the Children's Hospital, University of Cologne, Germany, or via cooperating centers. | n=24Experimental: n=12Age (months)mean (SD): 18.6 (3.2)Control: n=12Age (months)mean (SD): 19.4 (3.2) | Experimental: WBV 12–22Hz, 2.5mm, in three exercises: (A) standing still or alternately squatting and standing up, (B) sitting on the platform and (C) “four point position” (9min, 10/wk×14 weeks)+Regular physical therapyControl: Regular physical therapy | Gross motor function=GMFM-66Activities of daily living=PEDI in the self-care and mobility domainsCognitive Development=Bayley II Mental ScaleMedium term effects=14 wksFollow-up=14 wks | Galileo® system combined with a tilt table=sinusoidal vibration, alternately on the right and left side |
| Ward et al.49 2004 | Pre-or post-pubertal neurologic disabled, ambulant children, boys and girls, indicated by consultant community pediatricians. | n=20Experimental: n=10Age (yr)mean (SD): 6.9 (2.4)Control: n=10Age (yr)mean (SD): 11.2 (4.7) | Experimental: WBV 90Hz, standing (10min/day, 5/wk×24 wks)Control: standing on the platform without vibration (10min/day, 5/wk×24 wks) | Spine and tibial BMD, diaphyseal cross-sectional bone area, periosteal bone circumference, polar moment of inertia, cortical thickness, and muscle area=3-D QCT scannerMedium term effects=24 wks | Vertical ground-based vibration |
| Musculoskeletal conditions | |||||
| Högler et al.53 2017 | Children with mild to moderate osteogenesis imperfecta, boys and girls, recruited from specialist clinics at tertiary Children's Hospitals in Birmingham, Sheffield and Manchester, as well as through an advertisement placed on the Brittle Bone Society website. | n=24Experimental: n=12Age (yr)mean: 9.38Control: n=12Age (yr)mean: 6.49 | Experimental: WBV, 20–25Hz, 2–4mm, knees bent (10–45°, semi-squat and squat position (18min, 2/day×20 wks)+Regular physical therapyControl: Regular physical therapy | Lean mass and BMD=DXATibial BMD=pQCT scannerWalking distance=6-min walking test (6MWT)Medium term effects=20 wks | Galileo M™=Side-to-side alternating vertical sinusoidal (rotational) vibrations around a fulcrum in the mid-section of the plate. |
| El-Shamy52 2017 | Boys with hemophilia type A, selected from Al-Noor Hospital, Makkah, Saudi Arabia. | n=30Experimental: n=15Age (yr)mean (SD): 12.22 (2.33)Control: n=15Age (yr)mean (SD): 11.90 (2.74) | Experimental: WBV, 30–40Hz, 2–4mm, semi-squats (12–15min, 3/wk×12 wks)+Conventional physical therapy program (1h/d, 3/wk, 12 wks)Control: Conventional physical therapy program (1h/d, 3/wk, 12 wks) | Muscle strength (knee extension)=Isokinetic dynamometerFemur and lumbar spine BMD=DXAWalking distance=6-min walking testShort term effects=12 wks | Power Plate Next Generation Vibration Platform=simultaneously oscillates in the vertical, anterior–posterior, and mediolateral planes, predominantly vertical vibration |
| Burn-related conditions | |||||
| Edionwe et al.54 2016 | Severely burned children (≥30% total body surface area burns), boys and girls, recruited immediately after discharge from the hospital. | n=19Experimental: n=9Age (yr)mean (SD): 11.7 (3.7)Control: n=10Age (yr)mean (SD): 13.1 (4.0) | Experimental: WBV, 30–40Hz, 2–4mm, semi-squats (12–15min, 5/wk×6 wk)+Progressive resistance exercise (3/wk) and aerobic conditioning (30min to 1h, 5/wk)Control: Progressive resistance exercise (3/wk) and aerobic conditioning (30min to 1h, 6/wk) | Lean body mass, BMD and BMC=DXAMuscle strength (knee extension and flexion)=isokinetic dynamometerShort term effects=6 wks | Power Plate Next Generation=vibration platform simultaneously oscillates in the vertical, anterior–posterior and mediolateral planes |
SD, standard deviation; WBV, whole body vibration; wk, week; wks, weeks; DXA, dual-energy X-ray absorptiometry; BMD, bone mineral density; BMC, bone mineral content; vBMD, volumetric bone mineral density; TUG, timed up and go test; GMFM, gross motor function measure; PEDI, pediatric evaluation disability inventory.
Mean methodological quality of included trials was 5.5 out of 10 on the PEDro scale (scores ranged from 4.0 to 8.0). Methodological issues included: absence of concealed allocation (n=8 studies, 53%); absence of report of similar groups at baseline (n=2 studies, 14%); blinding of therapist (n=0 study, 0%), participant (n=0 study, 0%) and assessors (n=5 studies, 33%); more than 15% dropouts (n=7 studies, 53%); and intention-to-treat analysis (n=5 studies, 33%). Detailed assessment of methodological quality of included trials is in Table 2.
PEDro scores of included trials (n=15).
| Trial | Random allocation | Concealed allocation | Groups similar at baseline | Participant blinding | Therapist blinding | Assessor blinding | Adequate follow-up | Intention to-treat analysis | Between group difference | Point estimate and variability | Total (0-10) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Edionwe et al.54 2016 | Y | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Eid20 2015 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| El-Shamy and Mohamed21 2012 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| El-Shamy46 2014 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| El-Shamy52 2017 | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Erceg et al.51 2015 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| González-Aguero et al.50 2013 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Hogler et al.53 2017 | Y | Y | Y | N | N | N | N | N | Y | Y | 5 |
| Ibrahim et al.23 2014 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Ko et al.47 2016 | Y | N | N | N | N | N | Y | N | Y | Y | 4 |
| Lee and Chon22 2013 | Y | Y | Y | N | N | Y | Y | N | Y | Y | 7 |
| Matute-Llorente et al.55 2015 | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Ruck et al.48 2010 | Y | Y | Y | N | N | N | N | N | Y | Y | 5 |
| Stark et al.42 2016 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Ward et al.49 2004 | Y | N | N | N | N | Y | Y | Y | Y | Y | 6 |
| Total | 15 | 7 | 13 | 0 | 0 | 5 | 8 | 5 | 15 | 15 | 5.5/10 |
N, no; Y, yes.
Investigated participants included children with neurological (cerebral palsy21–23,42,46–49 and Down syndrome20,50), metabolic (overweight),51 musculoskeletal (hemophilia52 and osteogenesis imperfecta53) and burn-related conditions.54 Average age ranged from 15.8 months50 to 18.6 years old.42
InterventionOnly three trials investigated the effect of WBV compared to minimal intervention49–51 and twelve trials investigated additional effects of WBV compared to other intervention.20–23,42,46–48,52–55 Trials used different vibrating platforms and dosage parameters. Platform frequencies ranged from 522 to 90Hz49 and amplitude was less than 6mm. WBV programs were static (duration per session ranged from 5 to 20min or dynamic (duration per session ranged from 3 to 20min, two to five times per week, with total duration ranging from three to twenty four weeks).
OutcomesThe investigated primary outcomes included those related to body structure and function (i.e. lean body mass, bone mineral density, knee strength extension and overall stability) and secondary outcomes those related to activity and participation (i.e. gait speed, walking distance, gross motor function, self-care and mobility). All trials reported short- and medium-term effects only.
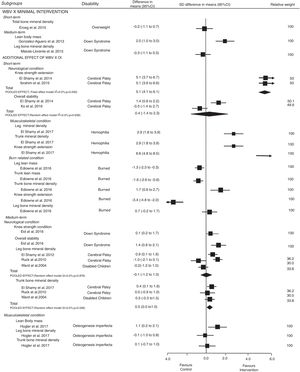
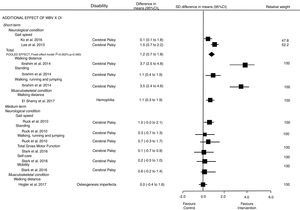
Effectiveness of whole body vibrationEffects of WBV compared with minimal intervention and its additional effects to other intervention were analyzed separately by health condition, and results were presented by the ICF's domains: Fig. 2 presents the results for outcomes related to the body structure and function domain (our primary outcomes); and Fig. 3 presents the results for outcomes related to the activity and participation domains (our secondary outcomes).
Only one trial including 24 children with Down syndrome50 showed a large effect of WBV on lean body mass at medium-term follow-up when compared to minimal intervention, SMD was 2.0 (95% CI 1.0–3.0). Other two trials including overweight participants51 and Down syndrome55 showed no effects on total bone mineral density and leg bone mineral density at short- and medium-term follow-up, respectively. SMDs were respectively: −0.2 (95% CI −1.1 to 0.7)51 and −0.3 (95% CI −1.1 to 0.5).55 Current evidence showing a large effect of WBV on lean body mass at medium-term in children with Down syndrome is very-low quality. Furthermore, evidence that additional effect of WBV does not increase total and leg bone mineral density at short- and medium-terms in overweight participants and Down syndrome was very-low as well.
Additional effects of whole body vibration compared to other interventionNeurological conditionBody structure and function impairment (Fig. 2): pooled estimates showed no additional effects of WBV on leg bone mineral density at medium-term follow up; SMD was −0.1 (95% CI −1.2 to 1.0). However, pooled estimates for trunk bone mineral density showed additional effect at medium-term follow up; SMD was 0.6 (95% CI 0.1–1.0). For knee strength extension, the pooled estimates [SMD=5.1 (95% CI 4.1–6.1)] and SMD from one study 0.1 (95% CI 0.2–1.7) showed additional effects of WBV on short- and medium-term follow up, respectively. Taken together, the evidence was low quality and indicated that additional effect of WBV provides a medium effect size on trunk bone mineral density, and low and large effect on knee strength extension at short and medium-term follow-up, respectively. SMD from one study showed additional effects of WBV on overall stability 1.4 (95% CI 0.6–2.1) at medium-term follow up, but the pooled estimates did not show significant effects at short-term follow up; SMD was 0.4 (95% CI −1.4 to 2.3). The strength of the evidence ranged from low to very-low quality and indicated a large effect size that additional effect of WBV improve overall stability at medium-term follow up but not in short.
Activity and participation limitation (Fig. 3): Pooled estimates showed additional effects of WBV on gait speed at short-term follow up; SMD was 1.2 (95% CI 0.7–1.8). However, one study did not find a positive effect of WBV on gait speed at medium term follow up; SMD was 1.0 (95% CI −0.0 to 2.1). The evidence, which ranged from low to very-low quality, indicated that additional effect of WBV provides a large effect on gait speed at short-term follow up compared to other interventions. SMDs from individuals studies investigating cerebral palsy showed a positive effect of WBV on walking distance 3.7 (95% CI 2.4–4.6), standing 1.1 (95% CI 0.4–1.9) and running, walking and jumping gross motor function domains 3.5 (2.4–4.6) at short-term follow up. However, the SMDs from individual studies did not find any effect at medium-term follow up for total gross motor function 0.1 (95% CI −0.7 to 0.9), self-care 0.2 (95% CI −0.5 to 1.0), mobility 0.6 (95% CI −0.2 to 1.4), standing 0.3 (95% CI −0.7 to 1.3) and walking, running and jumping 0.7 (95% CI −0.3 to 1.7) at medium-term follow-up. Taken together, the strength of the evidence was very-low quality that additional effects of WBV provides a large effect on walking distance and gross motor function domains at short-term follow up.
Musculoskeletal conditionBody structure and function impairment (Fig. 2): SMDs from individual studies showed a large effect of additional effect of WBV on lean body mass 1.1 (95% CI 0.2–2.1) in children with osteogenesis imperfecta at medium-term follow-up. For leg (SMD=2.9 [95% CI 1.8–3.9]; −0.1 [95% CI −1.0 to 0.8]) and trunk bone mineral density (SMD=2.9 [95% CI 1.8–3.9]; 0.1 [95% CI −0.7 to 1.0]) the studies showed a large effect of WBV at short-term (hemophilia) and medium-term (osteogenesis imperfecta) follow-up, respectively. One study showed no effect of WBV on knee strength extension in children with hemophilia at short-term follow up; SMD was 6.6 (95% CI 4.8–8.5). The quality of evidence was very low.
Activity and participation limitation (Fig. 3): SMDs from individual studies showed a large effect of additional effect of WBV on walking distance 1.1 (95% CI 0.3–1.9) at short term follow up in children with hemophilia, but no effect 0.0 (95% CI −0.4 to 1.8) at medium term follow-up in children with osteogenesis imperfecta compared to other interventions. The quality of evidence was very low.
Burn-related conditionBody structure and function impairment: SMD from one study showed a large effect of additional WBV on leg −1.3 (95% CI −2.3 to −0.3) and trunk lean mass −1.6 (−2.6 to −0.6), and trunk bone mineral density 1.7 (95% CI 0.6–2.7), but not for knee strength extension −3.4 (95% CI −4.8 to −2.0) and leg mineral density 0.7 (95% CI −0.2 to 1.7) in burned children at short-term follow-up. The quality of evidence was very low.
Sensitivity analysisThe sensitivity analysis investigated the impact of poor methodological quality (removing trials with PEDro score <5 out of 10) and health condition (removing heterogeneous disabilities). Methodological quality suggested impact on trunk bone mineral density [SMD=0.3 (−0.3 to 1.0)], but not on leg mineral density [SMD=−0.7 (95% CI −1.5 to 0.0)] at medium-term follow up, and knee strength extension [SMD=5.1 (95% CI 3.6–6.6)], overall stability [SMD=−1.4 (95% CI −2.2 to −0.6)] and gait speed [SMD=−1.4 (95% CI −2.2 to −0.6)] at short term-follow up in children with neurological conditions. Health condition sensibility analysis showed impact on trunk bone mineral density [SMD=0.5 (95% CI −0.0 to 1.1)], but not for leg mineral density [SMD=−0.1 (95% CI −2.1 to 1.9) at medium-term follow up in children with neurological condition.
DiscussionThis review aimed to summarize the current evidence of the effects of WBV, alone or combined, on deficiencies and limitations in children and adolescents with disabilities at short and medium-term follow-up. The estimates results were obtained from three studies comparing WBV versus minimal intervention and twelve studies comparing additional effect of WBV versus other interventions. In general, WBV compared to minimal intervention either showed no significant benefit in deficiencies of body structure and function, or the quality evidence was very-low quality to be clinically worthwhile. Pooled results indicated that additional effect of WBV provides a low to high effect size on deficiencies and activity limitations compared to other interventions, but these trials showed very-low to low quality evidence.
It has been speculating that WBV stimulates a moving pattern which activates spinal neurons leading to compensatory muscle contraction in leg and trunk.56 The muscle performance benefit is probably caused by neurogenic pathways potentiation involving spinal reflexes and muscle activation, called tonic vibration reflex.57,58 The mechanism causing strength and power increases from acute vibration is still being debated. However, the current proposal is that vibration provides a mechanical stimulus that causes the muscle fibers to stretch, thereby evoking a natural stretch, which enhances the neuromuscular function through neurogenic excitability and recruitment.57,59,60 Thus, this neural activation are related to muscle improvement, similar to those neural changes seen after conventional resistance and power training.57,61 Then, WBV training have been associated with increased muscle strength in disabled children with neurological and musculoskeletal conditions.53 Beyond vibration training (vertical or rotation) to be able to improve peak muscle forces (mechanical loading), increase of bone mass are expected probably due to the mechanostat theory.56,62 The WBV approach would serve as an anabolic stimulus impeding bone loss in children with neurologic, musculoskeletal and burn-related conditions.21,49 In another way, the high-frequency mechanical stimuli of the WBV which are generated by a vibrating platform stimulate sensory receptors50 also may be considered appropriate to improve the overall stability in neurological conditions.20
Although secondary outcomes such as gait capacity (gait speed and walking distance) and gross motor function (standing dimension and walking, running and jumping dimension) improved in children with neurologic conditions,22,23,48 the extent of its effect and the underlying mechanism are unknown. The increase of primary outcomes like bone mass and muscle strength may be associated.56,63
Due to small number of studies, the selected studies compared WBV with a large range of other interventions (i.e., progressive resistance exercise, aerobic conditioning and conventional physical therapy), disabilities (i.e., cerebral palsy, Down syndrome, osteogenesis imperfecta, hemophilia, overweight and burned children) and protocols settings. Despite the comparison of different outcomes and protocols, the trials typically showed no significant difference in outcomes between the groups, or a small to high effect in favor of WBV, but the high effect sized found was too wide. Furthermore, the sensitivity analysis of the present study (i.e., by health condition and methodological quality) revealed no impact on the majority of the outcomes, except for trunk bone mineral density in children with neurological conditions. Taking together, the findings limits to include some clinical worthwhile effects.
Previous systematic review33 showed effects of WBV on health-related physical fitness (body composition, cardiorespiratory fitness, flexibility, muscular strength, and muscular endurance) of children and adolescents with disabilities. Other systematic reviews also suggested positive effects of WBV in children with cerebral palsy,27,64,65 osteogenesis imperfecta,66 syndrome de down33 and Duchenne.67 Findings for some of the investigated outcomes of interest were not consistent with ours and a potential explanation is the methods. Previous studies did not follow PRISMA statement,35 they included low-quality studies (i.e. not randomized controlled trials),32,33 none effect size was estimated for clinical relevance of their findings32,33,64–66 they did not investigate the impact of poor methodological quality on the estimated effects,27,32,33,65,66 which compromises their clinical implications.
This present study throughout a high sensitive search strategy select eligible trials in all relevant databases, in accordance to Cochrane guidelines.35 Searches were also done by searching of potential trials from hand searching. Although, it is reasonable that some studies might have been published in local databases and, as a consequence, they were not selected in this study.68
Due this review included a small number of randomized trials the limited number of included studies and the high heterogeneity of conditions precluded pooling of data for the majority of the outcomes in this review. Futures high quality studies should clarify the impact of WBV (alone or combined) on deficiencies and limitations in children and adolescents stratified by disability type. Furthermore, studies should also evaluate the long-term effects of WBV, as well as investigate the impact of specific protocol settings, such as frequencies, amplitude and cumulative doses of WBV.
The present findings contribute to important information for clinicians, their patients and researchers. Enthusiasm with new expensive interventions suggesting improvements in the structure and body system in children and adolescent with disabilities need to be followed by scientific evidence.
ConclusionThis is the first systematic review with meta-analysis investigating effects of WBV in children and adolescents with disabilities. Current evidence is often very-low quality and recommendation of WBV in clinical practice is weak, mainly compared with minimal intervention. WBV combined with other intervention may improve some outcomes related to the ICF's body structure and function domain, and other outcomes related to the ICF's activity and participation domains short- and medium-terms. Further high-quality studies are warranted as they are likely to change current estimates.
Conflict of interestThe authors declare no conflicts of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.