Peripheral vascular disease (PVD) causes significant pain and disability in patients. Current conservative treatment for PVD is often limited to physical exercise. However, several recent studies have investigated the effects of physical therapy modalities in patients with PVD.
ObjectiveThis systematic review and network meta-analysis (NMA) aimed to compare the effects of different physical therapy modalities and physical exercise in improving the walking function of patients with PVD.
MethodsThis study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline, NMA extension. We searched six databases for relevant randomized clinical trials (RCTs) published between 2013–2023. The risk of bias was assessed using the Cochrane risk-of-bias tool for randomized trials version 2 (RoB2). MetaInsight and R were used to conduct the NMA.
ResultsWe analyzed 21 studies in the NMA. The results showed that shockwave therapy (SMD = 1.41, 95 %CI (0.58, 2.24)) and vacuum therapy (SMD = 0.72, 95 %CI (0.16, 1.29)) were effective independently in improving the walking function of patients with PVD. Combined hydrotherapy and exercise programs also performed better than exercise-only programs (SMD = 0.74, 95 %CI (0.38, 1.09)). While electrotherapy yielded a significant effect when performed independently (SMD = 1.43, 95 %CI (0.53, 2.33)), but was not effective when combined with exercise.
ConclusionOur findings suggest that shockwave and vacuum therapy can be used as a treatment for patients with PVD who have difficulties participating in physical exercise. Hydrotherapy could assist patients participating in physical exercise programs to achieve better outcomes.
This study was registered in the PROSPERO database CRD42023461442.
Peripheral vascular disease (PVD) is caused by limited blood supply in the lower limbs.1 Current evidence suggests that 5–6 % of the general population is affected by PVD.2 Patients with PVD are characterized by reduced ankle-brachial index (ABI)3 and leg pain during walking.4,5 The leg pain associated with PVD usually aggravates with physical exercise, and can be relieved by resting.6 Therefore, it is common for patients with PVD to have reduced mobility. Reduction in mobility not only affects quality of life, but also increases the risk of ulceration, gangrene, and amputation.5,7 Therefore, effective management to improve mobility in patients with PVD is essential. Currently, PVD can be managed through medications and surgries.6,8 There are also non-invasive alternatives such as physical therapy.9 Physical therapy can consists of exercises and physical activities. It can also consists of therapeutic modalities which include thermal, mechanical, electromagnetic, and light energies.10 At the moment, physical therapy for patients with PVD is focused on land-based exercises such as walking and use of a treadmill, as it can improve the circulation and prevent functional decline of the patients.4,11,12 However, not all patients with PVD are suitable for physical exercise. Older patients, patients with severe claudication, and patients with lower socio-economic status have difficulties participating in exercise programs.13 These barriers include fear of falling and the presence of co-morbidities such as severe arthritis or heart conditions.13
Several recent studies investigated the effects of physical therapy modalities such as hydrotherapy, vacuum therapy, electrotherapy, and shockwave therapy in patients with PVD.14–17 Compared to exercises, physical therapy modalities are passive interventions, which could be more appropriate for patients that have difficulties in performing basic walking exercises.14–16 In some studies, physical therapy modalities were combined with elements of physical exercise.17,18 However, which type of physical therapy modality, potentially provided in combination with an exercise program, is best suited for patients with PVD remains unclear. Therefore, in this study, we aim to (1) compare the effects of different physical therapy modalities and determine which type of modality was best suited for patients with PVD and (2) compare the effects of different physical therapy modalities with physical exercise programs. In this review, we define “physical therapy modalities” as any treatment that involves the use of physical agents. Therapies that involved exercise and the use of physical agents, such as walking exercise in water, were analyzed as combined therapy with components of therapeutic modality and exercise. To compare the effects of multiple therapies and combined therapy, we used a network meta-analysis (NMA), which allowed us to simultaneously compare the effects of multiple interventions.
MethodsThis systematic review and NMA was registered in the PROSPERO database (CRD42023461442). The review team followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.19
Database search and study selectionTo identify randomized controlled trials (RCTs) that investigated the effects of physical therapy modalities or physical exercise programs in patients with PVD, we searched six databases: PubMed, Medline, Web of Science, Embase, Cochrane Trials, and PEDro. To reflect the contemporary development of treatment for PVD, we only searched studies published in the last 10 years (January 2013-September 2023). In the database search, we used keywords relevant to PVD and its symptoms (peripheral vascular disease OR peripheral arterial disease OR intermittent claudication), different physical therapy modalities and physical exercise (laser OR heat OR hydrotherapy OR aquatic therapy OR water based OR crenotherapy OR shockwave therapy OR extracorporeal shockwave therapy OR electrical stimulation OR electrotherapeutic OR compression OR intermittent pneumatic compression OR vacuum therapy OR exercise OR physical activity OR walking), and indexes for the mobility and walking function of patients with PVD (mobility OR walk distance OR 6-minute walking test OR absolute claudication distance OR initial claudication distance).
The inclusion criteria for this review included: (1) RCTs that included patients with PVD; (2) Interventions consisting of physical therapy modalities or physical exercise programs; (3) Studies that compared the effects of physical therapy modality or physical exercise program with a control group that did not involve either treatment, or studies that directly compared the effects of a physical therapy modality with a physical exercise program; (4) Outcome measurement included assessment of walking function of patients with PVD, and (5) articles needed to be written in English. Studies with the following characteristics were excluded: (1) Studies with a high risk of bias; (2) Studies that were follow-up analyses of existing RCTs.
The search results were saved in a citation manager (Endnote 20, Clarivate, Philadelphia, PA, USA). Two reviewers (GCZ & JHO) independently screened the titles and abstracts to select potentially eligible studies for full-text review. When the two reviewers had different opinions regarding the eligibility of a study, consensus was achieved by discussion with a third reviewer.
Assessment of the quality of methodology and risk of biasThe Cochrane risk-of-bias tool for randomized trials version 2 (RoB2) was used to assess the methodological quality of the included studies. Studies were graded as having “low”, “some concerns”, or “high” risk of bias.20 Studies with “high” risk of bias were excluded from this review. The assessment was performed independently by two reviewers (GCZ & JHO). When there were differences regarding the assessment results of a study, the two reviewers would discuss and reach a consensus. If a consensus could not be reached, a discussion with a third reviewer (CHH) was made until a consensus was achieved.
Data extraction and synthesisWe extracted data from each RCT following the recommendation of the Cochrane collaboration. The author's name, study location/country, sample size, mean age of the participants, percentage of women, Fontaine stage of the participants, intervention received by the participants, outcome measurement, and findings of each study were extracted.21 The PlotDigitizer was used to extract data from studies that presented data in figure format only.22 Authors of studies that did not include sufficient data for meta-analysis were contacted as well. For NMA, the mean and standard deviation of relevant outcomes were extracted. If the article reported the results using other formats (e.g., median and interquartile range), mean and SD were estimated following the guidelines from the Cochrane collaboration.21 The extracted data were verified by all three reviewers.
Statistical analysisTo compare the effects of different physical therapy modalities and physical exercise programs in improving the walking function of patients with PVD, we performed a random effect-frequentist NMA using MetaInsight23 and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). Using NMA instead of traditional meta-analysis methods allowed us to compare the efficacy of multiple interventions such as the different physical therapy modalities, physical exercise programs, and other controlled interventions. The effect sizes of different interventions were presented using standardized mean difference (SMD) and 95 % confidence interval (CI) to accommodate different outcome measurements used in each study. A network plot was used to present the geometry of the treatment network, different interventions were presented as nodes, and connected nodes indicated that the two treatments were directly compared. The number of studies that made direct comparisons were shown as numbers on the line connecting two nodes. Potential inconsistency between the results of NMA and direct pairwise comparison was evaluated by the inconsistency index.21 Possible publication bias was assessed by inspecting the symmetry of funnel plots and the result of the Egger’s test.
ResultsStudy identification and selectionThe initial search identified 7060 articles. After removing duplicates, the remaining 6257 studies were screened, and 194 studies investigating the effects of physical therapy modalities or physical exercises in patients with PVD were selected for full-text review. Among these 194 studies, 172 were excluded either because (1) the study was not an RCT, (2) it did not assess the walking function of patients with PVD, or (3) it did not include a control group or compared the effects of physical therapy modality with physical exercise. The remaining 22 studies were included in this review, and except for one study that did not have sufficient data for meta-analysis,24 all 21 studies were included in the NMA (Fig. 1).
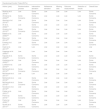
Methodological quality and risk of biasAmong the 22 included RCTs, three were graded “low” risk of bias.25–27 While the remaining 19 studies were graded as having “some concerns”. The most common risk was that the study could not mask the patients from the intervention they received, all 19 studies that were graded “some concerns” had this issue. However, it is difficult to avoid this limitation in studies that compared different physical therapy treatments. All three reviewers agreed that the risk of bias in these 19 studies did not significantly affect the validity of these studies but were natural limitations in the study design. The results of the RoB2 assessment are summarized in Table 1.
Methodological qualities of included studies.
| Randomized Control Trials (RCTs) | |||||||
|---|---|---|---|---|---|---|---|
| Author (year) | Randomization process | Intervention deviation | Adherence deviation | Missing data | Outcome measurement | Selection of results | Overall bias |
| Afzelius et al. (2018)14 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Ahmed et al. (2022)28 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Akerman et al. (2019)35 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Ali et al. (2022)15 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Alvarez et al. (2015)24 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Babber et al. (2020)38 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Breu et al. (2014)29 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Embrey et al. (2017)16 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Hackl et al. (2017)25 | Low | Low | Low | Low | Low | Low | Low |
| Hageman et al. (2020)26 | Low | Low | Low | Low | Low | Low | Low |
| Kapusta et al. (2022)36 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Koelemay et al. (2022)33 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Lamberti et al. (2016)34 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| McDermott et al. (2013)30 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
| McDermott et al. (2017)27 | Low | Low | Low | Low | Low | Low | Low |
| McDermott et al. (2018)31 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| McDermott et al. (2019)39 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Novakovic et al. (2019)40 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Paldan et al. (2021)32 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Park et al. (2019)17 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Park et al. (2020)18 | Low | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Quarto et al. (2017)37 | Some Concerns | Some Concerns | Low | Low | Low | Low | Some Concerns |
Note: The risk of bias in included studies was assessed using the Risk of Bias tool for randomized trials version 2 (RoB2).
Among the 22 included studies, six were conducted in the U.S., two each in Egypt, Germany, Netherlands, Italy, and Korea. The remaining six studies were conducted in Austria, Denmark, Poland, Slovenia, New Zealand, and the U.K. These studies were published between 2013 and 2022, with sample sizes ranging from 22 to 240, and the mean age of the participants ranging from 51.2 years to 75.3 years. Most studies had more men than women. One study only included men.28 Two studies only included women.17,18 Two studies did not specify the percentage of men/women in the study population.16,29 For the severity of PVD in the study population, 10 studies did not specify the Fontaine stage of the study participants. While most (n = 11) of the remaining 12 studies included patients with mild to moderate PVD (Fontaine stages I to III). Only one study specifically included patients with more severe PVD (Fontaine stage III-IV).24 The characteristics of the included studies are summarized in Table 2.
Characteristics of the included studies.
Notes: 6MWD, 6-minute walking distance; ACD, absolute claudication distance; CG, control group; Ex., exercise; GMCSF, Granulocyte-Macrophage Colony-Stimulating Factor; IG, intervention group; IPC, intermittent pneumatic compression; Max. Walk. Dist., maximum walking distance; N/A, data not available; NS, not specified; PWT, peak walking time a = This study did not specify the number of female participants & mean age for each group;.
Most (n = 19) of the 22 included studies were of two-arm design. Four studies compared a control group to a physical therapy modality: laser acupuncture,28 intermittent pneumatic compression (IPC),29 electrotherapy,25 and vacuum therapy.26 Three studies compared the effects of physical exercise programs with control groups that did not receive active treatment,30–32 and two studies compared the effects of physical exercise programs with surgical revascularization.33,34 The remaining 10 two-arm studies directly compared the effects of a physical therapy modality with a physical exercise program. Among these studies, five compared the effects of hydrotherapy with exercise programs with exercise-only programs17,18,35–37; two compared the effects of electrotherapy with exercise programs with exercise-only programs16,38; and one each compared the effects of vacuum therapy,14 shockwave therapy,15 and IPC program with physical exercise programs.24 There were also three multi-arm studies included in this review: (1) the study by McDermott et al.27 was a 4-arm study with a group of combined granulocyte-macrophage colony-stimulating factor (GMCSF) and exercise treatment, a placebo plus exercise group, a GMCSF-only group, and a placebo-only group); (2) another study by McDermott et al.39 was a 3-arm study that had two exercise groups that received aerobic or resistance exercise programs and a control group that received usual care; and (3) the study by Novakovic et al.40 had two groups that received either a moderate-pain exercise program or a pain-free exercise program, and a control group that received usual care. For the analysis, the data from the three studies27,39,40 exercise groups and the GMCSF-only and placebo-only groups of McDermott et al.27 were combined using Cochrane’s formula.21 The treatment provided for each intervention and control groups of each study are summarized in Table 2. A detailed description of the dose and frequency of each study’s interventions is shown in the Supplementary table S1. The treatment network of the aforementioned interventions is shown in Fig. 2.
Measurements of walking functionHalf (n = 11) of the included studies used the 6-minute walk test (6MWT) to evaluate the walking function (distance walked in 6 min) of patients with PVD. The measure is under the influence of PVD and the patient’s cardiopulmonary function. The other two common measurements for walking function were the absolute claudication distance (ACD) (n = 4) and maximum walking distance (n = 5), both of which measure the maximum distance a patient can walk before the patient is forced to stop.41 These two tests primarily tested the walking endurance of the patients. The remaining two studies used peak walking time24 and distance walked in the previous week.31 The peak walking time primarily measured the patients’ endurance in walking, while the distance walked in the previous week measured the walking functional capacity of patients. The outcome measurements of the included studies are summarized in Table 2.
Effects of physical therapy modalities and physical exercise programs in improving walking function of patients with PVDIn total, 21 studies were included in the NMA. The treatment network of different interventions is shown in the network plot (Fig. 2). The results of the NMA are shown in the lower triangle of the league table (Table 3). The results showed that compared to control groups, shockwave therapy (SMD = 1.41, 95 %CI (0.58, 2.24)), hydrotherapy with exercise (SMD = 1.09, 95 %CI (0.68, 1.50)), vacuum therapy (SMD = 0.72, 95 %CI (0.16, 1.29)), and physical exercise (SMD = 0.35, 95 %CI (0.09, 0.62)) were effective in improving the walking function of patients with PVD. Among these, shockwave therapy yielded significantly better effects compared to physical exercise (SMD = 1.06, 95 %CI (0.27, 1.85)). Combined hydrotherapy and exercise programs also yielded significantly better effects than exercise-only programs (SMD = 0.74, 95 %CI (0.38, 1.09)). On the other hand, electrotherapy was effective independently (SMD = 1.43; 95 %CI (0.53, 2.33)), but when combined with exercise programs, electrotherapy did not yield significant effects compared to controlled intervention (SMD = 0.60; 95 %CI (−0.10, 1.29)). There was no significant inconsistency between the effect sizes estimated by the NMA and the head-to-head comparison, and no significant publication bias was shown in our NMA.
League tables of the effects of different treatments on the walking function of patients with peripheral vascular disease.
Note: The estimated effect size was presented as standardized mean differences (SMD) and 95 % confidence interval (CI). IPC, intermittent pneumatic compression; Shockwave, shockwave therapy.
Treatment comparisons that showed significant effects are in bold.
The results of our NMA showed shockwave and vacuum therapy, compared to control, were effective in improving the walking function of patients with PVD. Furthermore, shockwave therapy yielded better effects than physical exercise programs. Whereas combined hydrotherapy and exercise programs have better effects compared to exercise-only programs. While electrotherapy yielded significant effects when performed independently it failed to yield significant effects when combined with exercise programs.
Improving the ability to walk is an important aspect of caring for patients with PVD.42 In current clinical practice, exercise was often the only conservative treatment for patients,4,11 which greatly limited treatment options for patients with PVD. Through this study, we showed that shockwave therapy and vacuum therapy could be effective in improving walking function for patients with PVD. The severe leg pain that often limited the ability to walk in patients with PVD is usually caused by reduced blood supply to the leg.4 Therefore, treatments that can improve the circulation of lower limbs is potentially effective for improving walking function of patients with PVD. Current clinical management for PVD reflected this, as many of the current treatments for PVD were aimed at improving lower limb circulation.4,5
Shockwave therapy is a non-invasive therapy that was used in managing intermittent claudication in patients with PVD.43–45 Current evidence suggests that shockwave therapy can improve the circulation of its affected area by facilitating tissue revascularization and promote angiogenesis, and, therefore mitigate the negative effects of ischemia such as pain associated with PVD.46,47 Several non-randomized studies also showed that shockwave therapy can improve the maximum walking distance (MWD) and pain-free walking distance (PFWD) of patients with PVD.44 Our findings reflected the findings in previous studies, as shockwave therapy yielded significantly better effects compared to the control groups and traditional physical exercise. However, only one study investigated the effect of shockwave therapy in this review. The study had some concerns regarding its methodological quality, especially in its randomization process. Therefore, further evaluation of the effect of shockwave therapy in patients with PVD using large, multicenter RCT is still needed.
Vacuum therapy was also shown to produce significant effect in improving the walking function of patients with PVD. The basic concept of vacuum therapy is using intermittent negative pressure generated by a vacuum chamber to apply pressure on arteries and veins that supply the lower limbs and therefore improve circulation, mitigate the symptoms of PVD, and improve the physical function of patients.26 The studies that assessed the effects of vacuum therapy also have overall good methodological quality and a larger sample size.14,26 Therefore, we believe that vacuum therapy is likely to be effective in improving walking function in patients with PVD. However, whether that effect is directly associated with improved local circulation requires further examination, as whether vacuum therapy can directly improve local circulation in patients with PVD is still unclear.
We also found that electrotherapy is effective independently in improving the walking function of patients with PVD, yet when combined with exercise, electrotherapy failed to yield significant effect. This contradictory finding may be the result of limited number of studies, as there was only one study that investigated the independent effect of electrotherapy in patients with PVD, and only two investigated the effects of combined electrotherapy and exercise program. Therefore, currently we cannot determine whether electrotherapy is appropriate, with or without exercise program, for improving the walking function of patients with PVD.
Lastly, hydrotherapy combined with exercise was shown to improve walking function in patients with PVD in this review. Furthermore, the effects of combined hydrotherapy and exercise is significantly better compared to exercise-only programs. This finding suggested that hydrotherapy may enhance the effects of physical exercise programs in improving the walking function of patients with PVD.
LimitationsA limitation of this study is that there is significant heterogeneity in the number of studies that investigated the effect of each intervention. For example, there was only one study that investigated the effect of laser acupuncture in patients with PVD,28 while there were 5 that investigated the effect of hydrotherapy. Also, we analyzed the data of the included studies using the reported post-treatment data only and did not include intra and intergroup differences to minimize potential errors in the manipulation of data. This may affect the interpretation of results in this NMA. Moreover, in this review, most studies that utilized hydrotherapy and electrotherapy performed their treatments in conjunction with exercise programs, the independent effects of hydrotherapy and electrotherapy still need to be clarified in further studies. Another limitation is that the increased availability of personal electronic devices has been changing the way physical therapy is delivered and monitored.48–51 However, we did not find studies that evaluate the effect of technology based physical therapy delivery method in patients with PVD. Which could also be an important facet in future research. Lastly, in this review, we only used the keywords “exercise” and “physical activity” in the search for studies that investigated the effects of exercises in patients with PVD. In addition, our analysis did not investigate the difference in effect of different exercises because we analyzed the effect of exercises as one intervention. Therefore, we cannot identify the difference in effect of different types of exercise programs.
ConclusionThe findings of this study suggested that shockwave and vacuum therapy, when compared to control, effectively improved the walking function of patients with PVD, and the effects of shockwave therapy were significantly better compared to physical exercise. Hydrotherapy, when performed in conjunction with physical exercise, could enhance the effects of physical exercise in patients with PVD.
The authors declare no competing interest.