Coronary artery disease (CAD) lead to cardiovascular autonomic control disfunctions that can worsen exercise and/or posture adjustments.
ObjectivesTo verify the cardiovascular responses to low-intensity isometric handgrip exercise performed in different postures in CAD patients. This study tested the hypothesis that the posture influences the cardiovascular responses during isometric handgrip exercise and that the presence of CAD leads to greater cardiovascular stress during this type of exercise.
MethodsWe investigated cardiovascular responses to isometric handgrip exercise in 15 CAD patients (CADG) and 15 health matched-control (CG). The subjects performed isometric handgrip exercise at 30% of maximum voluntary contraction until exhaustion in SUPINE, SITTING and STANDING positions. Systolic arterial pressure, diastolic arterial pressure, mean blood pressure, heart rate, peripheral vascular resistance, cardiac output, stroke volume and double product were measured during rest (baseline), exercise (peak value) and recovery in the 1st minute (REC1). Delta PB (ΔPB, peak minus baseline) and PR1 (ΔPR1, peak minus REC1) were calculated.
ResultsHigher ΔPB and ΔPR1 of systolic and mean arterial pressure and double product were observed in STANDING when compared to SITTING and/or SUPINE. CADG showed higher ΔPB of systolic and mean arterial pressure in all postures and higher ΔPR1 of strove volume in the SITTING.
ConclusionWe concluded that the posture during isometric handgrip exercise influences the cardiovascular responses with STANDING leading to higher cardiovascular stress. CAD promoted higher arterial pressure responses however these responses were physiological and expected due to the presence of disease and type of exercise.
Coronary artery disease (CAD) can lead to impaired cardiovascular autonomic control responses to stimuli such as exercise and postural changes.1,2 To avoid or reduce these impairments, exercise-based cardiac rehabilitation is imperative to CAD patients. Combined aerobic and resistance exercises are, currently, important cardiac rehabilitation components to maintain the myocardial and peripheral muscle functional capacity.3–5
Among resistance exercises, isometric exercises are recommended3,4 but they are little prescribed in cardiac rehabilitation because possible high-pressure responses and considerable sympathetic modulation increase.6,7 However, these responses were observed in high intensity exercises, which is not feasible for cardiac patients.6,7 Recently, low-intensity isometric handgrip training has become an important tool for reducing resting arterial pressure in hypertensive.4,8–10 However, acute cardiovascular responses to this type of low-intensity exercise are not explored in CAD patients who are the most frequent category of patients in cardiac rehabilitation.
Acute cardiovascular responses to isometric handgrip exercise are dependent on intensity, duration and muscle mass involved,11,12 which should be adjusted based on the patient’s clinical status and cardiac tolerance. Moreover, other factors, such as posture during exercise, can influence cardiac responses.13–15 Orthostatic stimulus maneuver, which induces sympathetic stimulation, has been used to evaluate cardiovascular autonomic control. Thus, evaluating these responses to low-intensity isometric exercise performed in different postures might advance our knowledge about the autonomic nervous system regulation in CAD patients as well as the possibility of applicability of isometric exercise in CAD and its benefits.
The understanding of these responses is important to guide and help health professionals to set the correct and safe intensity of isometric handgrip exercise and clarify the possible benefits. Thus, the objective of the present study is to verify the cardiovascular responses to low-intensity isometric handgrip exercise performed in different postures in CAD patients. We hypothesized that posture during isometric exercise influences these responses and that the presence of CAD promotes greater cardiovascular stress when compared to a control-matched group.
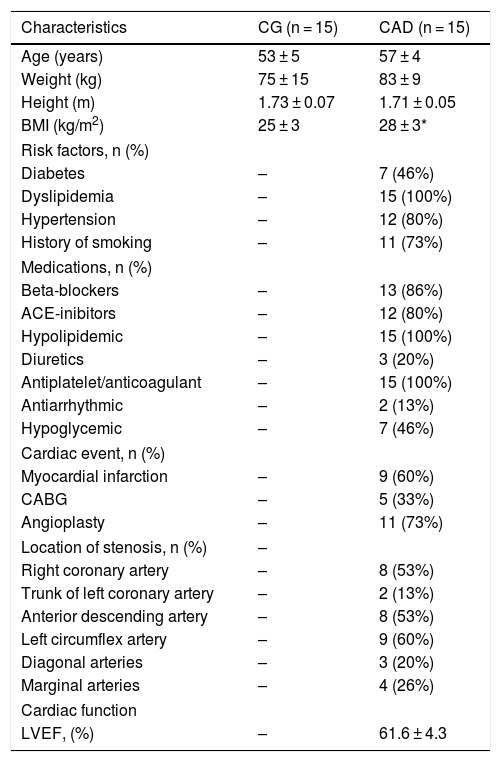
MethodsStudy populationFifty-six subjects were eligible into the laboratory database. Twenty-six subjects were excluded: 1 CAD patient was excluded by abnormalities in clinical exercise test; 23 subjects (19 CAD patients and 4 CG) did not attend the clinical exercise test and/or the first visit. Thirty-two men were evaluated (Table 1): 17 patients with CAD (CADG group) and 15 control subjects (CG). After experimental protocol, 2 CAD patients were excluded due to invalid data. All patients of the CAD group had a diagnosis confirmed by coronary angiography examination. The clinical evaluation for the enrollment in this study took place at least 6 months after the event (coronary angioplasty, myocardial infarction or coronary artery bypass grafting). Subjects of CG group were characterized as healthy according to the results of a clinical assessment and biochemical blood analysis.
Characteristics, medications, clinical and angiographic data.
| Characteristics | CG (n = 15) | CAD (n = 15) |
|---|---|---|
| Age (years) | 53 ± 5 | 57 ± 4 |
| Weight (kg) | 75 ± 15 | 83 ± 9 |
| Height (m) | 1.73 ± 0.07 | 1.71 ± 0.05 |
| BMI (kg/m2) | 25 ± 3 | 28 ± 3* |
| Risk factors, n (%) | ||
| Diabetes | – | 7 (46%) |
| Dyslipidemia | – | 15 (100%) |
| Hypertension | – | 12 (80%) |
| History of smoking | – | 11 (73%) |
| Medications, n (%) | ||
| Beta-blockers | – | 13 (86%) |
| ACE-inibitors | – | 12 (80%) |
| Hypolipidemic | – | 15 (100%) |
| Diuretics | – | 3 (20%) |
| Antiplatelet/anticoagulant | – | 15 (100%) |
| Antiarrhythmic | – | 2 (13%) |
| Hypoglycemic | – | 7 (46%) |
| Cardiac event, n (%) | ||
| Myocardial infarction | – | 9 (60%) |
| CABG | – | 5 (33%) |
| Angioplasty | – | 11 (73%) |
| Location of stenosis, n (%) | – | |
| Right coronary artery | – | 8 (53%) |
| Trunk of left coronary artery | – | 2 (13%) |
| Anterior descending artery | – | 8 (53%) |
| Left circumflex artery | – | 9 (60%) |
| Diagonal arteries | – | 3 (20%) |
| Marginal arteries | – | 4 (26%) |
| Cardiac function | ||
| LVEF, (%) | – | 61.6 ± 4.3 |
Values are expressed as mean ± standard deviation or as number of subjects (n) and percentage (%).CG: control group; CADG: coronary artery disease group; BMI: body mass index; ACE: angiotensin converting enzyme; CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction.
Inclusion criteria were non-smoking, non-habitual drinking or no drug use, absence of neurological and pulmonary disease and non-physical limitations to perform the exercise protocol. For the CADG, only patients with preserved left ventricular ejection fraction (≥58%), confirmed by coronary angiography and/or echocardiography, were included.16 For both groups, only subjects who performed less than 150 min of physical activity per week were included.17
The exclusion criteria were: subjects with ECG abnormalities in clinical exercise test (ST segment depression greater than 2 mm, ventricular and supraventricular arrhythmias, sustained supraventricular tachycardia or non-sustained atrial tachycardia, atrial fibrillation and atrioventricular blocks), body mass index (BMI) greater than 35 kg/m2, hypertension or diabetes mellitus not controlled, non-completed evaluations or invalid data (BeatScope and/or LabChart softwares).
All subjects were informed about the purposes and procedures and, after agreeing, they signed a written consent form. The study was conducted in accordance with the Declaration of Helsinki for medical research involving human subjects and was approved by the Ethics Committee of Universidade Federal de São Carlos, São Carlos, SP, Brazil (number: 311.260/CAAE: 13287613.0.0000.5504).
Clinical exercise testAll individuals performed a maximum clinical exercise test with a cardiologist to check the health condition and exclusion criteria cited above. Heart rate, arterial pressure and electrocardiogram responses were evaluated during exercise. The clinical exercise test was performed with treadmill and modified Bruce protocol was utilized.18
Experimental protocol and cardiovascular measuresThe subjects were instructed to avoid caffeinated beverages as well as moderate or heavy exercise on the day before the protocols. The temperature (22–24 °C) and humidity (40–60%) of the room were controlled and all the experiments were carried out in the afternoon.
ECG was acquired from the lead II using the BioAmp FE 132 (ADInstruments, South Wales, Australia), while the arterial pressure was recorded by a finger plethysmographic device (Finapres Medical Systems, Amsterdam, Netherlands). A thoracic belt (Marazza, Monza, Italy) was used to monitor the respiratory activity through the movements of the thorax. The arterial pressure signal was calibrated in each session by regularly measuring the blood pressure with a sphygmomanometer from the equipment. The arm was maintained at heart level in all positions.
Heart rate values were derived from the interval between two R-waves of the ECG through the software LabChart Pro (ADInstruments). Systolic arterial pressure, diastolic arterial pressure, mean arterial pressure, peripheral vascular resistance, cardiac output and stroke volume were obtained from the arterial pressure waves and analyzed in the software Beat Scope® Easy (Finapress Medical Systems).19,20 The double product was the product of systolic arterial pressure and heart rate. The data were recorded during 10 min of baseline (average of beat-to-beat values recorded during the 60 s before the onset of the exercise), peak (the highest value recorded during the contraction) and 10 min of recovery. Delta PB (peak minus baseline) and PR1 (peak minus REC1) were calculated. REC1 was the value at the 1st minute of recovery.
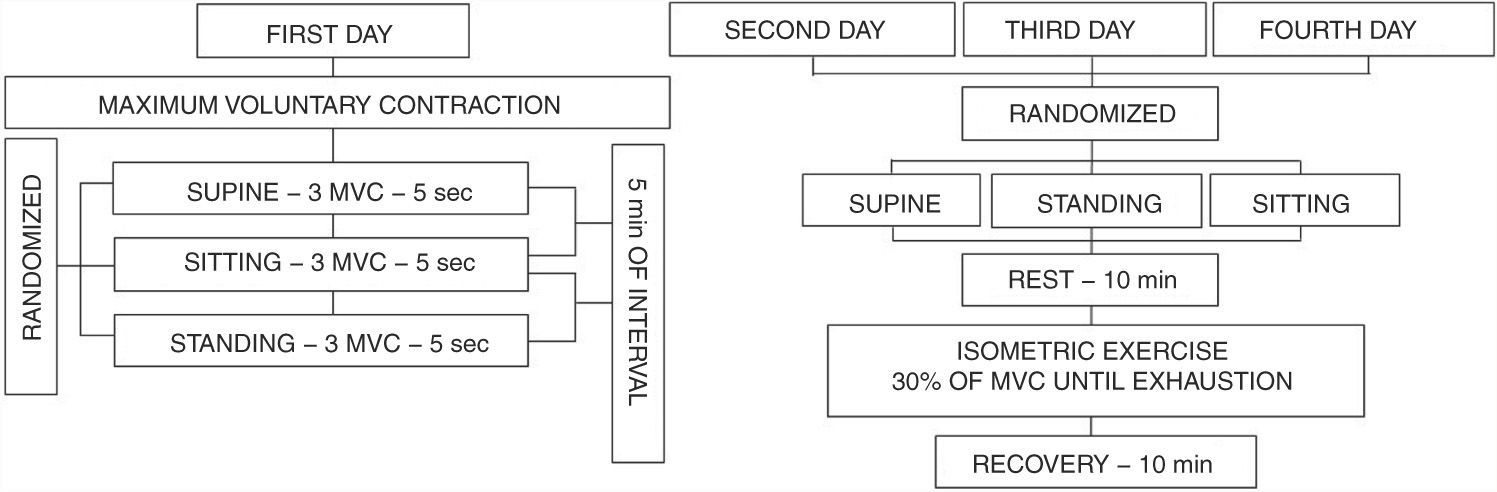
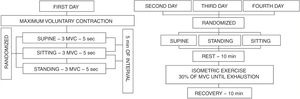
Isometric handgrip exercise protocolThe maximum voluntary contraction (MVC) and its percentages were performed using an analogue handgrip dynamometer (Jamar® - Sammons Preston, INC Bolingbrook, IL, USA) with the dominant hand. The position of the elbow was maintained at 90° with the arm parallel to the body. On the first day the subjects performed 3 MVC sustained for 5 s, with 5-min of recovery between them. The MVC value was determined as the highest value obtained of the three measures with less than 10% of variation between them. The three subsequent visits consisted of sustained submaximal contraction until exhaustion performed at 30% of MVC in SUPINE, SITTING and STANDING positions, that were randomized (Fig. 1). The interval between the visits were at least 48 h and no more than 168 h (1 week). The percentage of 30% was selected because it was the stimulus most used in the literature.8–10,13,21–25
Visual feedback through a mirror and verbal encouragement were given by the same evaluator to ensure the maintenance of intensity until exhaustion. Subjects were instructed not to perform Valsalva Maneuver to avoid changes in heart rate and arterial pressure.26 The occurrence of Valsalva Maneuver was confirmed by the presence of reflex bradycardia, which was detected by heart rate analysis. If Valsalva Maneuver occurred, the contraction was repeated.
Statistical analysisBased on systolic blood pressure from a previous study,21 a sample size for the current study was determined to be 11 subjects per group to provide statistical power (β = 0.8 and α = 0.05). Considering a dropout rate of 30%, we evaluated 15 subjects per group. Normality of the data distribution was verified by the Shapiro-Wilk test. The unpaired Student’s t-test was used to compare age and anthropometrics variables between the groups. Two-way analysis of variance (ANOVA) with repeated measures was used to assess changes in delta (PB and PR1) responses, time contraction and force (MVC and 30% of MVC) between the groups and postures. The data were analyzed using a commercial statistical program (Sigmaplot, ver.11.0, Systat Software, San Jose, CA, USA) and p < 0.05 was considered statistically significant.
ResultsTable 1 describes the characteristics of groups under study. There were no differences between age, weight and height between the CG and CADG, except for the body mass index. Regarding force values and time contraction, no differences were observed between the groups and postures. The values of 30% of MVC were 12 (± 2) kgf for CG in all postures and 10 (± 1) kgf for CADG in all postures. The 30% of MVC time contraction were 123 (± 28) vs 112 (± 21) vs 117 (± 24) sec in CG and 130 (± 20) vs 107 (± 27) vs 119 (± 35) sec in CADG (SUPINE vs SITTING vs STANDING).
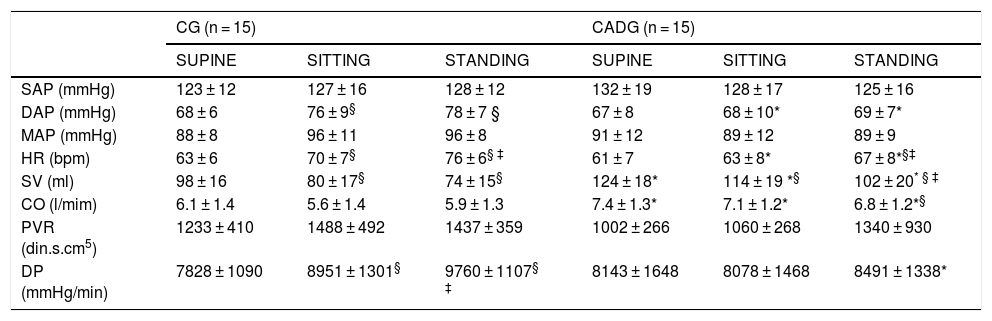
Table 2 describes the cardiovascular parameters during baseline. Higher diastolic arterial pressure (CG), heart rate (CG and CADG) and double product (CG) were observed in SITTING and/or STANDING when compared to SUPINE. As well, lower stroke volume (CG and CADG) and cardiac output (CADG) were observed in SITTING and/or STANDING when compared to SUPINE. CADG showed higher stroke volume, cardiac output, double product (in STANDING) and lower diastolic arterial pressure and heart rate (in SITTING and STANDING). No differences were observed for systolic and mean arterial pressure and peripheral vascular resistance during baseline.
Baseline cardiovascular parameters.
| CG (n = 15) | CADG (n = 15) | |||||
|---|---|---|---|---|---|---|
| SUPINE | SITTING | STANDING | SUPINE | SITTING | STANDING | |
| SAP (mmHg) | 123 ± 12 | 127 ± 16 | 128 ± 12 | 132 ± 19 | 128 ± 17 | 125 ± 16 |
| DAP (mmHg) | 68 ± 6 | 76 ± 9§ | 78 ± 7 § | 67 ± 8 | 68 ± 10* | 69 ± 7* |
| MAP (mmHg) | 88 ± 8 | 96 ± 11 | 96 ± 8 | 91 ± 12 | 89 ± 12 | 89 ± 9 |
| HR (bpm) | 63 ± 6 | 70 ± 7§ | 76 ± 6§ ‡ | 61 ± 7 | 63 ± 8* | 67 ± 8*§‡ |
| SV (ml) | 98 ± 16 | 80 ± 17§ | 74 ± 15§ | 124 ± 18* | 114 ± 19 *§ | 102 ± 20* § ‡ |
| CO (l/mim) | 6.1 ± 1.4 | 5.6 ± 1.4 | 5.9 ± 1.3 | 7.4 ± 1.3* | 7.1 ± 1.2* | 6.8 ± 1.2*§ |
| PVR (din.s.cm5) | 1233 ± 410 | 1488 ± 492 | 1437 ± 359 | 1002 ± 266 | 1060 ± 268 | 1340 ± 930 |
| DP (mmHg/min) | 7828 ± 1090 | 8951 ± 1301§ | 9760 ± 1107§ ‡ | 8143 ± 1648 | 8078 ± 1468 | 8491 ± 1338* |
Values are expressed as mean ± standard deviation. CG: control group; CAD: coronary artery disease; SAP: systolic arterial pressure; DAP: diastolic arterial pressure; MAP: mean arterial pressure; HR: heart rate; SV: stroke volume; CO: cardiac output; PVR: peripheral vascular resistance; DP: double product.
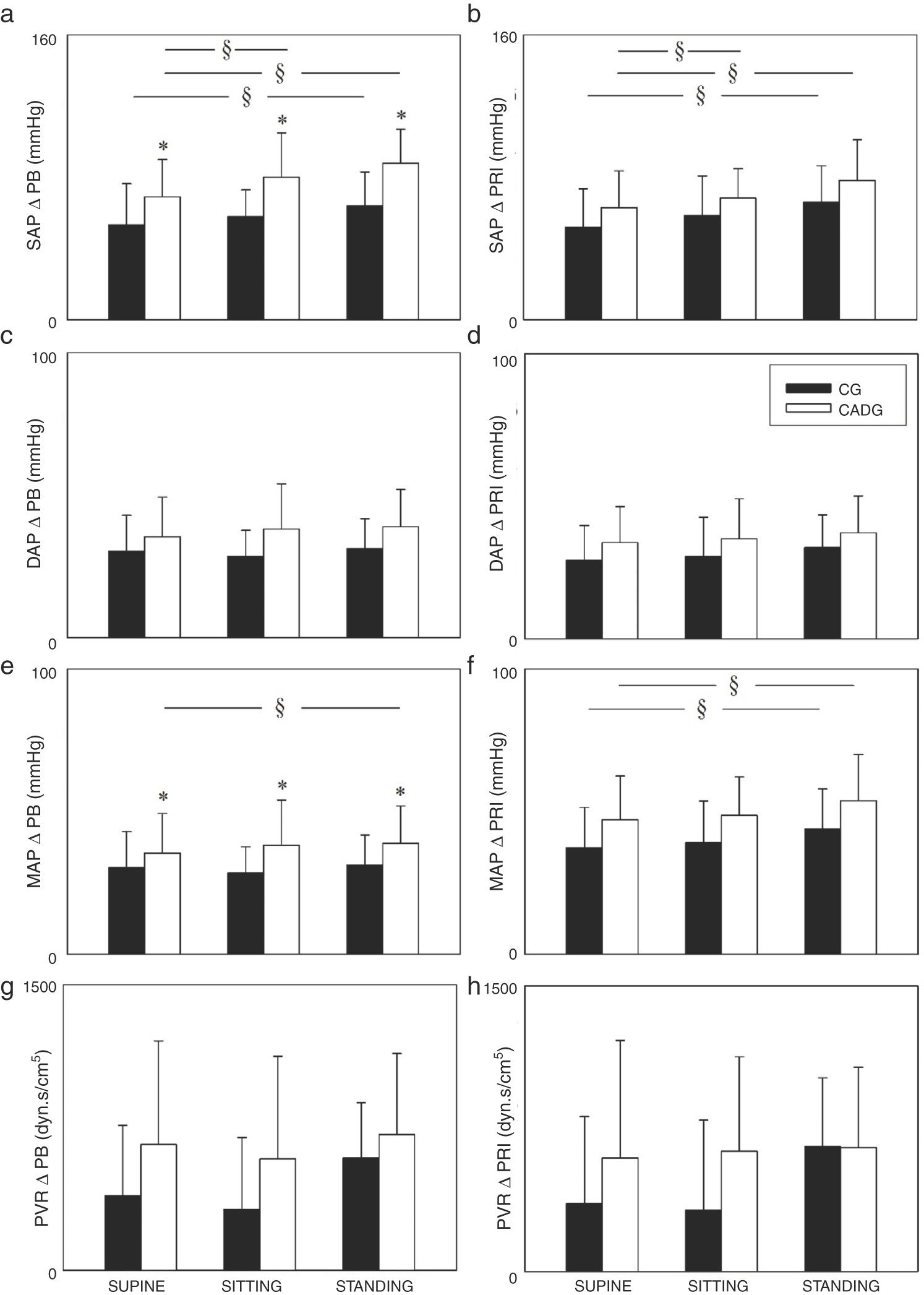
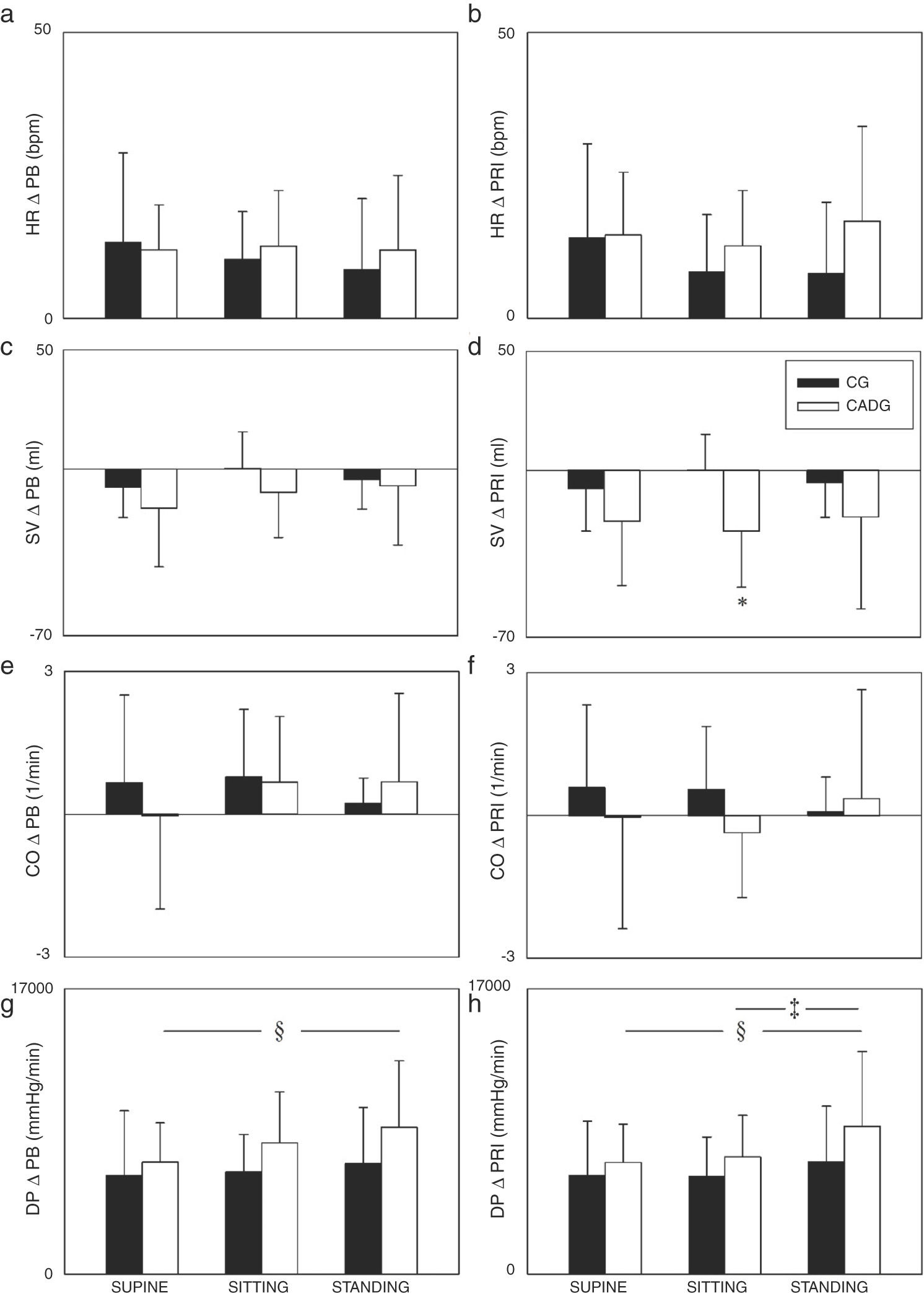
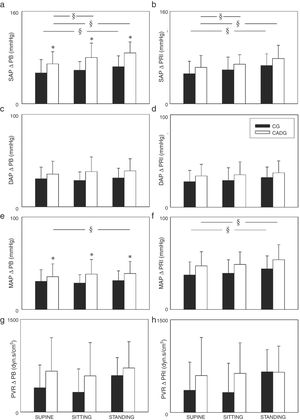
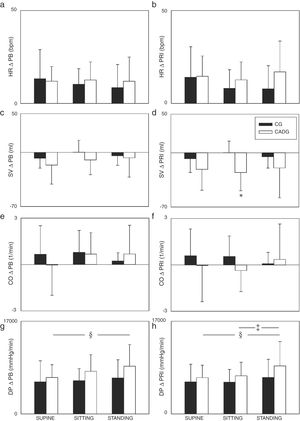
The Figs. 2 and 3 show the PB and PR1 deltas. Regarding systolic and mean arterial pressure, higher ΔPB (Fig. 2a/e) and ΔPR1 (Fig. 2b/f) were observed in STANDING when compared to SITTING and/or SUPINE, except for the mean arterial pressure (ΔPB) in CG. CADG showed higher ΔPB of systolic and mean arterial pressure (Fig. 2a/e) in SUPINE, SITTING and STADING. Moreover, higher ΔPR1 of stroke volume (Fig. 3d) was observed in CADG in SITTING. No differences between were observed in diastolic arterial pressure (Fig. 2c/d), peripheral vascular resistance (Fig. 2g/h), heart rate (Fig. 3a/b), cardiac output (Fig. 3e/f) and double product (Fig. 3g/h).
Values are expressed as mean ± standard deviation. CG: control group; CAD: coronary artery disease; ΔPB: delta peak-baseline; ΔPR1: delta peak-rec1; SAP: systolic arterial pressure; DAP: diastolic arterial pressure; MAP: mean arterial pressure; PVR: peripheral vascular resistance; *p < 0.05 vs CADG; §p < 0.05 vs SUPINE.
The main findings of this study are about the influence of posture on cardiovascular responses during isometric handgrip exercise in CAD patients. First, in STANDING, higher arterial pressure responses were observed when compared to SITTING and/or SUPINE. Second, despite the presence of CAD, physiological responses were observed, similarly to CG.
Regarding baseline parameters (Table 2), CADG showed lower diastolic arterial pressure, heart rate and double product. Probably these findings are related to the medications normally used by CAD patients. Beta blocker medication, used by 13 of 15 patients, decreases the cardiac chronotropic and ionotropic responses, resulting in lower heart rate27 and antihypertensive medications, used by 12 of 15 patients, reduces peripheral vascular resistance e consequently decrease diastolic arterial pressure.28 Higher stroke volume and cardiac output were observed in CADG. We believe that these findings might be explained by the reduced heart rate, provide by beta blocker to maintain an adequate cardiac output or by the modelflow algorithm used to estimate cardiac output. The modelflow algorithm was previously validated during dynamic exercise29,30 but it may vary by 19% when compared to thermodilution method.31 However, although stroke volume and cardiac output are higher in CADG, it is important to emphasize that they are within a normal range.
During baseline STANDING, higher heart rate and double product and lower stroke volume and cardiac output were observed in CG and/or CADG, demonstrating the influence of posture on cardiovascular responses.13,14,21 It is known that STANDING promotes cardiovascular settings to maintain the circulatory demand required by the action of gravity.15,21 The upright position leads to a shift of blood volume to the lower body regions. To maintain a normal blood pressure, sympathetic stimulation promotes heart rate and peripheral vascular resistance increase through regulatory baroreflex mechanisms and increase of sympathetic vascular tone, respectively.14,15,21,32,33 Because of STANDING maintenance, there is a decline in venous return which might explain the progressive decrease of stroke volume and cardiac output, as observed in baseline.
Higher arterial pressure (systolic and mean arterial pressure) responses were observed in STANDING for both groups (Fig. 2), which confirm the influence of posture on cardiovascular responses during isometric handgrip exercise. We found few studies which assessed the cardiovascular responses in different postures during isometric handgrip exercise and the findings are varied among them. Legramante et al.13 and Iellamo et al.21 evaluated mild hypertensive patients and healthy subjects, respectively. The results from their studies are unlike ours because they did not observe difference between postures during isometric handgrip exercise. Both studies13,21 performed isometric handgrip for 2 min at 30% of MVC in supine, sitting and standing at the same day and always in this order, i.e., the position was not randomized and time recovery between the contractions was not shown.
On the other hand, Davey34 observed posture’s influence on QT interval in left ventricular hypertrophy and chronic heart failure patients. Hence, we are the first study to verify the influence of SUPINE, SITTING and STANDING on cardiovascular responses to isometric handgrip exercise in CAD patients. We believe that greater arterial pressure responses induced by STANDING might be an interaction between orthostatic adjustments added to isometric exercise reflex mechanisms, inducing a “double and integrative sympathetic stimulation”. Orthostatic adjustments were already discussed above. Regarding isometric exercise reflex mechanisms, cardiovascular responses are controlled by central command28,35 and exercise pressor reflex.36 As we know, vagal withdrawal and sympathetic stimulation, derived by central command, occurs at exercise onset to increase heart rate and arterial pressure. However, during isometric handgrip exercise, heart rate and arterial pressure rises are not proportionally similar as during dynamic aerobic exercise,35,37 as showed by us in Figs. 2 and 3.
During isometric handgrip exercise, there is a muscle mechanical action on the blood vessels due to the muscular action without joint movement.35 It promotes decrease of active skeletal muscle blood flow with intramuscular pressure increase, leading to metabolites accumulation, responsible for metaboreflex activation, through III and IV fibers, and consequent increase of sympathetic activity.7,35–37 Thus, we reasoned that metaboreflex activation added to orthostatic maintenance, might explain the higher arterial pressure responses in STANDING.
Higher arterial pressure responses (systolic and mean arterial pressure), were observed in CADG, as expected by us. These findings might be explained by the morphological disfunctions provided by CAD to the heart and arterial system. In our opinion, two important issues should be raised to explain it. First, the presence of hypertension, intrinsic to the CAD, can provides over the years a left ventricular hypertrophy.38 Second, the atherosclerotic process is systemic and can lead to increase of aortic arterial stiffness.22 Therefore, although we have not assessed whether these variables are modified, we reasoned that both issues cited above might be responsible to the afterload increase and consequent higher arterial pressure responses in CAD patients.
Despite these findings about higher arterial pressure responses in CADG, we consider that they showed adequate physiological and expected responses to isometric handgrip exercise. According to some authors,39,40 arterial pressure responses until 260 × 120 mmHg are acceptable for exercise maximal tests. We proposed a low-intensity exercise (30% of MVC) and the highest ΔPB observed in CADG, were 88 (± 18) (for systolic arterial pressure) and 58 (± 16) (for mean arterial pressure) in STANDING. Considering that systolic and mean arterial pressure baseline values in STANDING were 125 (± 16) and 89 (± 8), respectively, values close to the maximum were not achieved even with contractions until exhaustion.
It is important to highlight that we proposed low-intensity contraction until exhaustion to investigate the cardiovascular behavior during isometric handgrip exercise. However, in cardiac rehabilitation, exercise series are time limited and not until exhaustion. So, with shorter contraction time, lower arterial pressure responses will be reached. For this reason, we suggest the prescription of isometric handgrip exercise for CAD patients for endurance training and/or for arterial pressure modulation training. We believe that, for an endurance strength training, SUPINE posture seems to be most interesting because it resulted in lower cardiovascular stress. On the other hand, STANDING posture can be interesting for an arterial pressure modulation training because it resulted in higher systolic and mean arterial pressure delta responses and consequently, stimulate more responses from baroreceptors.
Similarly, other authors have also encouraged the isometric handgrip exercise prescription because they observed adequate cardiovascular responses in CAD patients.23–25,41 We proposed a low-intensity isometric exercise (30% of MVC) because it is the most used in literature, but other percentages can be tested as future perspectives. Moreover, to evaluate cardiovascular responses during contractions not until exhaustion seems to be to interesting and promising to improve the isometric handgrip training prescription for CAD patients and to search long term benefits.
In summary we encourage the application of low-intensity isometric handgrip exercise in cardiac rehabilitation for patients under conditions like those presented in this study, i.e, clinically stable patients (with controlled arterial hypertension, stable CAD, absence of arrhythmias and use of drugs) and without performing Valsalva maneuver. It is important to understand the physiological adjustments caused by CAD and to know about the isometric handgrip exercise characteristics to prescribe the safe intensity, that must be adjusted individually to avoid arterial pressure increases outside the safe range of CAD patients. Moreover, beyond the reasons cited above, it has low cost, easy applicability and accessibility for therapists and patients.
Finally, it is important to highlight that our study have some limitations, such as indirect measures of cardiac output, stroke volume and peripheral vascular resistance and use of beta blocker and/or antihypertensive medications by CAD patients. For these reasons, we tried to minimize both influences, i.e., medications and non-invasive measures, presenting cardiovascular delta values31 and not absolute values, which reflects the variations from baseline and/or recovery.
ConclusionsThe findings of present study conclude that STANDING position provides higher cardiovascular stress during isometric handgrip exercise. CAD promotes higher arterial pressure responses (systolic and mean arterial pressure) however these responses were physiological and expected due to the clinical disfunctions provided by disease. Thus, low-intensity isometric handgrip exercise can be prescribed in cardiac rehabilitation safely with cardiovascular monitoring, without performing Valsalva maneuver and considering the patient’s clinical condition and CAD stability.
AcknowledgementsThis study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES [(CAPES/CSF/PVE process: 23038.007721/2013-41); (CAPES/PNPD process: 23038.006927/2011-92)]; by The São Paulo Research Foundation (FAPESP process: 10/52070-4) and by National Council for Scientific and Technological Development - CNPq (CNPq process: 311938/2013-2).
Conflicts of interestThe authors declare no conflicts of interest.