Some inspiratory muscle training protocols for patients with heart failure report the request of diaphragmatic breathing during inspiratory loaded breathing. However, it is unclear whether this condition modifies the chest wall volumes.
ObjectiveThe primary purpose was to evaluate chest wall volumes during inspiratory loaded breathing as well as during inspiratory loaded breathing associated with diaphragmatic breathing in patients with heart failure.
MethodsSixteen men with heart failure functional class I to III, aged 50(SD=7) years were evaluated. Volumes of the pulmonary rib cage, abdominal rib cage and abdomen, as well as other breathing pattern variables, were assessed by optoelectronic plethysmography during quiet breathing, inspiratory loaded breathing, and inspiratory loaded breathing associated with diaphragmatic breathing.
ResultsChest wall tidal volume significantly increased from quiet breathing 0.53(SD=0.14)L to inspiratory loaded breathing 1.33(SD=0.48)L and to inspiratory loaded breathing associated with diaphragmatic breathing 1.36(SD=0.48)L. A significant volume variation was observed on the three compartments (p<0.05 for all). During inspiratory loaded breathing associated with diaphragmatic breathing, patients showed increased abdominal volume compared to quiet breathing [0.28(SD=0.05) to 0.83(SD=0.47)L, p<0.001]; as well as from inspiratory loaded breathing [0.63(SD=0.23) to 0.83(SD=0.47)L, p=0.044]. No significant changes were observed between the two inspiratory loaded breathing conditions on the percentages of the contribution of each chest wall compartment for the tidal volume, respiratory rate, minute ventilation, and duty cycle.
ConclusionWhen inspiratory loaded breathing was associated with diaphragmatic breathing, a higher volume in the abdominal compartment was obtained without significant changes in other breathing pattern variables.
Patients with heart failure (HF) may present impairments in strength and endurance of respiratory muscles because of increased respiratory effort, which causes worsening dyspnea and limits daily activities of living.1–3 Therefore, inspiratory muscle training has been suggested to improve respiratory muscle function, especially when inspiratory muscle weakness is identified.4–7 Besides the increase in inspiratory muscle strength, benefits were observed in diaphragm thickness,8 inspiratory muscle endurance,9–11 dyspnea,10,12,13 functional capacity, and quality of life.9,10,13,14
Different protocols for inspiratory muscle training (IMT) in heart failure were reported in clinical trials,8–14 with varying load levels and modulation, breathing pattern, frequency, and duration of training. The use of diaphragmatic breathing during inspiratory muscle training has also been reported in some studies.8–10,13,15 Similar improvements on maximal inspiratory pressure were found when IMT was associated or not associated with diaphragmatic breathing.4–7 However, none of these studies compared the results from these two different conditions.
To the best of our knowledge, only the study of Brandão et al.16 evaluated the distribution of chest wall volumes as well as of each compartment (pulmonary rib cage, abdominal rib cage, and abdomen) in patients with HF. The analysis was based on contributions of the right and left sides of the chest wall, during quiet breathing and inspiratory loaded breathing (ILB) compared with healthy controls. The authors observed that during ILB, patients showed a lower contribution of abdominal rib cage at both sides compared to controls.
Because abdominal displacement is influenced by the diaphragm,17 we hypothesized that patients could increase the abdominal volume when ILB is associated with diaphragmatic breathing, contributing to a higher chest wall volume. The primary purpose of this study was to evaluate chest wall volumes (total chest wall volume and the three compartments volumes of the chest wall) during ILB, as well as during ILB associated with diaphragmatic breathing (ILBdi) in patients with HF. Secondarily, the following variables were assessed: percentages of the contribution of the pulmonary rib cage, abdominal rib cage and abdomen; respiratory rate, minute ventilation, and duty cycle.
MethodsStudy design and sampleAn observational study was conducted in patients with HF recruited from the community. Patients (men) aged 30–59 years diagnosed with HF (left ventricular systolic dysfunction based on left ventricular ejection fraction<50%) were eligible.18 The inclusion criteria were as follows: clinical stability (no change in medications for the past three months); body mass index between 18.5 and 29.9kg/m2; no history of smoking; no obstructive pulmonary disease (forced expired volume in 1s/forced vital capacity>0.70, confirmed by spirometry)19; sedentary patients (do not practice at least 30min of moderate activity on most days of week); no history of unstable angina, myocardial infarction, or cardiac surgery within the previous three months. Participants were excluded if they were not able to perform the procedures or if they presented symptoms of fever or influenza during the assessment or for the seven days before the assessment. This study was approved by the Ethics Committee of the Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil (ETIC 0607.0.203.000-09), and all participants gave written informed consent.
Measurement instrumentsChest wall volumes and breathing patternThe optoelectronic plethysmography (OEP; BTS Bioengineering, Milan, Italy) was used to assess the chest wall and its three compartments volumes, as well as other variables of the breathing pattern: percentages of the contribution of the pulmonary rib cage, abdominal rib cage and abdomen; respiratory rate, minute ventilation and duty cycle. This system is a valid20–22 and reliable23 instrument to assess chest wall volumes and was already used during ILB,16 as well as during breathing exercises.24 In this study, a system with six cameras was used to register chest wall volumes by 89 markers around the trunk (37 anteriorly, 42 posteriorly, and 10 laterally), with the patient in the sitting position. The anatomical boundaries were the xiphoid appendix (between the pulmonary rib cage and the abdominal rib cage), the costal anterior margin and the lowest posterior point of the costal inferior margin (between the abdominal rib cage and the abdomen). Two calibration procedures were performed to correct the optical distortions and to provide the calculation of the 3D coordinates given a set of control points with known location. The details of technical procedures and calibration method are reported elsewhere.25Fig. 1 shows a model obtained during data collection. Markers are seen as points linked to obtain the three compartmental chest wall model.
Lung function, respiratory muscle function and physical activity levelLung function was assessed by a calibrated spirometer (Vitalograph® 2120, Ennis, Ireland). Patients performed up to eight forced maneuvers. The following variables were registered: forced expired volume in 1s, forced vital capacity, and, forced expired volume in 1s/forced vital capacity. Data were compared to predicted values according to Pereira et al.26
Inspiratory muscle strength was evaluated with an analogical pressure transducer (GeRar® Classe B, São Paulo, Brazil), and maximal inspiratory pressure was obtained during quiet breathing, after a deep inspiration from residual volume against an occluded airway with an air leak of approximately 2mm, according to ATS/ERS recommendations.27 The highest pressure of at least three measures (differences within 10%) was used for analysis and compared to predicted values.28
The Human Activity Profile was used to assess the physical activity level. It is a 94-item instrument that is valid to the Brazilian population29 and assesses the energy expenditure of different activities.30 Two scores are computed: maximum activity score, which represents the highest item value that the individual still makes, and adjusted activity score, which represents the highest item decreased by the number of items that the individual no longer makes. The physical activity level is classified as follows: sedentary=adjusted activity score<53; moderately active=53≤adjusted activity score≤74; active=adjusted activity score>74.
Inspiratory load deviceA threshold device (Threshold Inspiratory Muscle Trainer®, Healthscan Products Inc., NJ, USA) was used to perform ILB and ILBdi. This instrument provides resistance during inspiration, by linear and specific selected pressure, varying from 7 to 41cmH2O. The Threshold IMT is widely used for inspiratory muscle training in patients with heart failure.8–10,12–15
ProceduresMeasurements were performed in two days, with an interval of up to one week. On the first day, after the measurement of weight and height, the patients were evaluated by medical history, physical examination, lung function, inspiratory muscle strength, and physical activity level.
On the second day, patients were assessed by OEP at three conditions: quiet breathing, ILB, and ILBdi. During quiet breathing patients were instructed to breathe with their usual pattern. On the second condition – ILB – patients used a nose clip and were instructed to inspire through the mouth against a load of 30% of maximal inspiratory pressure,8,10 followed by a normal expiration: “take a deep breath and exhale as normal”. On the third condition – ILBdi – instructions were similar to the former (ILB) with emphasis on abdomen displacement: “take a deep breath emphasizing the expansion of the abdomen during inspiration and exhale as normal”. Each condition was performed during 3min. There was no formal instruction for the number of repetitions. Therefore, the respiratory rate was the one adopted by the patient. A rest period interval of at least 10min among conditions was observed until baseline parameters were attained. Heart rate and peripheral oxygen saturation were registered during all data acquisition, while blood pressure and effort sensation were verified before and after each of the three conditions, for monitoring. Measurements were performed by the same investigator.
Data reductionData acquisition was performed during a 3min period for each of the conditions. The second minute (steady-state) was analyzed.
VariablesThe following variables were analyzed by OEP: tidal volumes of the chest wall (Vcw), pulmonary rib cage (Vrcp), abdominal rib cage (Vrca), and abdomen (Vab); percentages of the contribution of the pulmonary rib cage (Vrcp%), abdominal rib cage (Vrca%), and abdomen (Vab%) for the tidal volume; respiratory rate, minute ventilation and duty cycle (Ti/Ttot).
Statistical analysisSample size was calculated based on a pilot study with the first 10 participants by using all analyzed variables. Considering a power of 0.80, an alpha of 5%, and the lowest effect size of difference between the three conditions (i.e. 0.364 for Vrca%), the highest sample size found was 14 participants.
Data were presented as measures of central tendency and dispersion. Normality was assessed by the Shapiro–Wilk test. Comparisons among the three conditions (quiet breathing, ILB, and ILBdi) were performed by analysis of variance (ANOVA) for repeated measures and the least significance difference (LSD) test was chosen as post hoc. A p<0.05 was considered statistically significant. The SPSS (version 17.0, IL, USA) was used for data analysis.
ResultsPopulation characteristicsTable 1 shows patients’ baseline characteristics. Initially, 26 patients were screened, and seven patients were found to be ineligible for the study (four presented pulmonary obstructive pattern on spirometry, two showed a left ventricular ejection fraction>50%, and one was obese). Three patients were excluded (two did not complete the protocol, and one presented 30% of maximal inspiratory pressure>41cmH2O). Thus, data from 16 patients were analyzed. Beta-blockers were used by 14 patients (94%), and 10 patients presented inspiratory muscles weakness and had a moderate physical activity level according to Human Activity Profile.
Characteristics of participants (n=16).
| Characteristics | X (SD) |
|---|---|
| Age (years) | 50.38 (7.01) |
| Weight (kg) | 71.53 (12.29) |
| Height (m) | 1.68 (0.07) |
| BMI (kg/m2) | 25.15 (2.97) |
| LVEF (%) | 33.88 (8.97) |
| FVC (% predicted) | 78.54 (18.43) |
| FEV1 (% predicted) | 75.02 (16.52) |
| FEV1/FVC | 0.78 (0.05) |
| MIP (cmH2O) | 74.56 (32.47) |
| MIP (% predicted) | 64.49 (27.50) |
| AAS (value) | 70.88 (16.46) |
Data presented as mean (X) and standard deviation (SD). BMI, body mass index; LVEF, left ventricular ejection fraction; FVC, forced vital capacity; FEV1, forced expired volume in 1s; FEV1/FVC, ratio of the forced expiratory volume in 1s to the forced vital capacity; MIP, maximal inspiratory pressure; AAS, adjusted activity score from Human Activity Profile.
Table 2 shows data related to chest wall volumes and breathing pattern variables during the three conditions (quiet breathing, ILB, and ILBdi). Vcw, respiratory rate, minute ventilation, and Ti/Ttot significantly increased from quiet breathing to ILB and ILBdi, with no difference between ILB and ILBdi. No significant changes were observed for Vrcp%, Vrca%, and Vab% among conditions.
Chest wall volume and breathing pattern data obtained during quiet breathing and inspiratory loaded breathing conditions (n=16).
| Variables | Conditions | ANOVA | ||
|---|---|---|---|---|
| Quiet breathing | ILB | ILBdi | p-Value | |
| Vcw (L) | 0.53 (0.46–0.61) | 1.33* (1.08–1.58) | 1.36* (1.10–1.61) | <0.001 |
| Vrcp% | 26.88 (22.10–31.62) | 32.55 (26.77–38.33) | 25.45 (17.59–33.29) | 0.113 |
| Vrca% | 18.56 (16.24–20.88) | 18.13 (14.34–21.91) | 14.88 (12.40–17.36) | 0.084 |
| Vab% | 54.56 (49.99–59.13) | 49.32 (41.54–57.10) | 59.67 (51.05–68.29) | 0.079 |
| RR (bpm) | 17.23 (14.86–19.61) | 11.78* (10.58–12.98) | 11.58* (10.09–13.07) | <0.001 |
| VE (L/min) | 8.77 (7.65–9.89) | 15.21* (12.63–17.79) | 15.09* (12.83–17.35) | <0.001 |
| Ti/Ttot | 41.21 (39.44–42.98) | 50.71* (45.70–55.73) | 49.90* (45.49–54.29) | <0.001 |
Data presented as mean (95% confidence interval). ILB, inspiratory loaded breathing; ILBdi, inspiratory loaded breathing associated with diaphragmatic breathing; Vcw, tidal volume of the chest wall; Vrcp%, percentage of the contribution of the pulmonary rib cage; Vrca%, percentage of the contribution of the abdominal rib cage; Vab%, percentage of the contribution of the abdomen; RR, respiratory rate; VE, minute ventilation; Ti/Ttot, duty cycle.
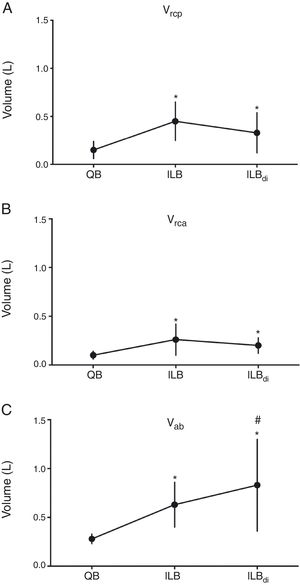
Fig. 2 shows data related to the three compartments volumes (Vrcp, Vrca, and Vab) during the three conditions. Significant increases from quiet breathing to ILB and to ILBdi were observed for all variables. Vrcp and Vrca showed no differences between ILB and ILBdi, whereas Vab significantly increased from ILB to ILBdi.
Volumes of chest wall compartments during quiet breathing, inspiratory loaded breathing and inspiratory loaded breathing associated with diaphragmatic breathing. QB, quiet breathing; ILB, inspiratory loaded breathing; ILBdi, inspiratory loaded breathing associated with diaphragmatic breathing. (A) Vrcp, volume of the pulmonary rib cage; (B) Vrca, volume of the abdominal rib cage; (C) Vab, volume of the abdomen. *p<0.05 (vs QB). #p<0.05 (vs ILB).
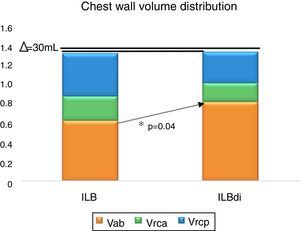
Fig. 3 shows the chest wall volume distribution comparison between the conditions ILB and ILBdi for each compartment. A non significant difference of 30mL was observed for Vcw (p>0.05). A significant change was observed only for Vab (p=0.04).
Chest wall volume distribution between ILB and ILBdi. ILB, inspiratory loaded breathing; ILBdi, inspiratory loaded breathing associated with diaphragmatic breathing. Vrcp, volume of the pulmonary rib cage; Vrca, volume of the abdominal rib cage; Vab, volume of the abdomen; Δ (delta), difference on the chest wall volume between ILB and ILBdi. *p>0.05 (ILB vs ILBdi, post hoc LSD).
The main findings of this study were as follows: (1) chest wall tidal volume significantly increased from quiet breathing to ILB and to ILBdi; (2) abdominal volume significantly increased during ILBdi with respect to ILB; (3) there were no changes between ILB and ILBdi in relation to chest wall volume, the percentage of compartments contribution, and breathing pattern variables.
The increase in the Vcw during ILB condition is consistent with results observed in healthy individuals31 or in patients with cardiopulmonary diseases.16,32 Da Gama et al.31 analyzed the acute effects of ILB (increasing loads until fatigue) on chest wall volumes, breathing pattern variables, and electromyographic activity of inspiratory muscles in 39 healthy individuals of both sexes. In men (n=19), it was observed that Vcw, Vrcp, and Vab significantly increased only after the load of 30cmH2O. In the present study, the selected load was 30% of the maximal inspiratory pressure, which corresponded to 22 (SD=10)cmH2O, and was probably correlated to a significant increase in Vcw. Considering that most people in the present study had inspiratory muscle weakness [maximal inspiratory pressure 74.56 (SD=32.47)cmH2O], loads greater than 20cmH2O already represent a greater percentage of resistance than that of the healthy individuals studied by Da Gama et al.,31 explaining the increase of Vcw.
The results of the present study are also similar to those observed by Brandão et al.,16 who found a significant increase of Vcw in both hemithorax of patients with HF, cardiomegaly, and inspiratory muscle weakness [maximal inspiratory pressure 45.94 (SD=15.17)cmH2O], when they performed an inspiratory load of 30% of maximal inspiratory pressure.
The diaphragmatic breathing consists of slow and deep inspirations performed with the priority displacement of the abdomen, aiming to improve ventilation at the lung bases.24,33 The contribution of the different compartments during diaphragmatic breathing was evaluated in 15 healthy subjects by Vieira et al.24 These authors found a higher contribution of the abdomen in comparison to rib cage. In the present study, diaphragmatic breathing was included in ILB, which limits comparison with the results of Vieira et al.24 However, a significant increase of Vab in ILBdi condition was observed when compared with ILB, indicating that patients with HF were able to mobilize a higher volume to the abdomen, possibly contributing to an improvement in the abdominal regional ventilation.
Ramsook et al.34 evaluated the electromyographic activity of inspiratory muscles of 10 healthy men during an ILB session (30 inspirations using POWERbreathe®, HaB International Ltd, UK) under two conditions similar to those of the present study. In the first condition, the participants performed the ILB session without guidance on the muscle recruitment strategy. In the second one, the participants were instructed to emphasize the anterior displacement of the abdomen during inspiration, that is, diaphragmatic breathing. The authors reported a significant increase in electromyographic activation of the diaphragm and in transdiaphragmatic pressure (measured by intraesophageal balloon) during the session performed with diaphragmatic breathing. Aliverti et al.17 pointed out that, among the three compartments of the chest wall, the abdomen volume variation is the one that best reflects the axial displacement of the cephalic margin of the diaphragm in the apposition zone. Thus, it is possible that the adoption of diaphragmatic breathing evidenced by the increase of Vab during ILBdi has contributed to emphasize the work of the diaphragm, whose function is frequently impaired in individuals with HF.1–3
There was no significant difference between ILB and ILBdi on the following variables: Vrcp%, Vrca%, Vab%, minute ventilation, respiratory rate, and Ti/Ttot. The inclusion of ILB, regardless of the muscle recruitment strategy used by the individual, seems to be the primary factor responsible for the breathing pattern changes.31 To overcome resistance, individuals breathe more deeply and increase inspiratory time, which promotes increased Vcw (in response to increased inspiratory lung volume) and, consequently, respiratory rate decreases, and minute ventilation and Ti/Ttot increase. The only study comparing two similar situations to ILB and ILBdi demonstrated that, in healthy individuals, the electromyographic activity of the scalene muscle was reduced in the ILBdi condition, whereas other non-diaphragmatic muscles maintained the same activity in this condition.34 These authors discussed that these muscles play a role in helping the diaphragm during ILB by generating the pressure necessary to overcome the resistance imposed by the equipment used. The need for rib cage muscles recruitment may explain the same contribution of each compartment to the volume between ILB and ILBdi observed in the present study, since the volume displaced by the abdomen alone would probably be insufficient to overcome the imposed load. We can hypothesize that it can also be related to lung properties changes previously reported in patients and animals with HF.35,36
To the best of our knowledge, this was the first study to evaluate chest wall volumes during ILB associated with diaphragmatic breathing in patients with heart failure. One of the limitations was the absence of a randomization between the ILB and ILBdi conditions and, therefore, it is not possible to exclude the learning effect during ILBdi. However, the non-randomization was intentional, since individuals could repeat the strategy of diaphragmatic breathing if it was the first task, which could influence the individual's inspiratory recruitment strategy. Another limitation was the lack of standardization of the inspiratory time and expiratory time during assessed conditions. Changes in respiratory rate and/or cadence (Ti/Ttot) may imply modifications in respiratory muscle performance,7 minimizing or optimizing muscular work against an inspiratory load.
In conclusion, our results showed that inspiratory loaded breathing with or without diaphragmatic breathing modifies chest wall volumes. The ILBdi increases abdominal volume, without influencing other breathing pattern variables. Future studies evaluating other aspects, such as chest wall volumes and compartments before and after an IMT period, and the energy expenditure and lung ventilation distribution, may contribute to clarify the results observed in the present study and providing greater benefits, especially with regard to diaphragmatic function in patients with HF.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES Grant 88881.068409/2014-01), Brazil. VFP is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – Grant 309990/2017-3) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Grant PPM-00373-17).