Young adults with patellofemoral pain (PFP) have a high prevalence of being overweight or obese, which is associated with impaired lower limb function and muscle weakness. However, the impact of being overweight or obese on pain sensitivity has not been explored.
ObjectivesWe investigated the association between body fat, skeletal muscle mass, and body mass index (BMI) with pressure hyperalgesia and self-reported pain in young adults with PFP.
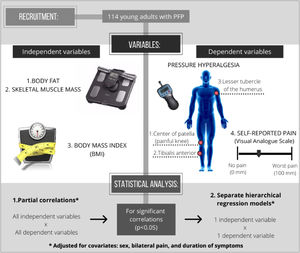
Methods114 adults with PFP (24 ± 5 years old, 62% women) were recruited. Demographics and self-reported pain (current and worst knee pain intensity in the previous month - 0–100 mm visual analog scale) were recorded. Body fat and skeletal muscle mass were measured using bioelectrical impedance. Pressure hyperalgesia was measured using a handheld algometer (pressure pain threshold) at three sites: center of patella of the painful knee, ipsilateral tibialis anterior, and contralateral upper limb. The association between body fat, skeletal muscle mass, and BMI with pressure hyperalgesia and self-reported pain were investigated using partial correlations and hierarchical regression models (adjusted for sex, bilateral pain, and symptoms duration).
ResultsHigher body fat and lower skeletal muscle mass were associated with local, spread, and widespread pressure hyperalgesia (ΔR2=0.09 to 0.17, p ≤ 0.001; ΔR2=0.14 to 0.26, p<0.001, respectively), and higher current self-reported pain (ΔR2=0.10, p<0.001; ΔR2=0.06, p = 0.007, respectively). Higher BMI was associated with higher current self-reported pain (ΔR2=0.10, p = 0.001), but not with any measures of pressure hyperalgesia (p>0.05).
ConclusionHigher body fat and lower skeletal muscle mass help to explain local, spread, and widespread pressure hyperalgesia, and self-reported pain in people with PFP. BMI only helps to explain self-reported pain. These factors should be considered when assessing people with PFP and developing their management plan, but caution should be taken as the strength of association was generally low.
Patellofemoral pain (PFP) is a common cause of knee pain in young adults, with an estimated annual prevalence of 22.7% in the general population.1 The diagnosis is predominantly clinical, with most people with PFP reporting a non-traumatic onset of pain around or behind the patella exacerbated by activities that load the patellofemoral joint.2 Symptoms are persistent, with one in two people continuing to experience pain 5–8 years after rehabilitation.3
Despite PFP being traditionally linked with abnormal loading of the patellofemoral joint,4–7 recent evidence indicate there is no direct relationship between pain and patellofemoral joint loading during stair ascent in women with PFP.8 Other factors, including the presence of pressure hyperalgesia (i.e. “increased pain from a stimulus that normally provokes pain”9), may play a greater role in the pain experience of people with PFP.10–13 Recent systematic reviews14,15 reported that people with PFP have lower pressure pain thresholds (PPT) in local (at the knee joint) and remote sites (such as tibialis anterior and lesser tubercle of the humerus) compared to non-injured people, indicating the presence of local, spread, and widespread pressure hyperalgesia.
Young adults with PFP also have higher body mass index (BMI) compared to non-injured controls.16,17 Correlations have been reported between measures of body composition and pressure hyperalgesia in chronic musculoskeletal conditions (i.e., increased BMI and body fat were associated with higher pressure hyperalgesia).18–21 Increased levels of pro-inflammatory cytokines by adipose tissue was previously reported in overweight populations,22 which may lead to sensitization of peripheral nociceptors and central nociceptive transmission pathways (i.e., peripheral and central sensitization, respectively).23 Increased levels of pro-inflammatory cytokines have also been longitudinally associated with reduced skeletal muscle mass,24 which may contribute to a sensitized profile. A recent study in a mixed-gender non-injured population reported that higher body fat and BMI were associated with lower PPT.25 The association between body composition measures (e.g., body fat, skeletal muscle mass, BMI) and pressure hyperalgesia has never been explored in young adults with PFP. A better understanding of how body fat, skeletal muscle mass, and BMI are associated with the pain experience in this population is likely to help guide both health care practitioners and researchers to improve the management of PFP (e.g., currently there is no trial targeting weight loss in PFP).26 Therefore, the aim of this study is to explore the association between body fat, skeletal muscle mass, and BMI with local, spread, and widespread pressure hyperalgesia and self-reported pain in young adults with PFP. Our hypotheses were that increased body fat and BMI and decreased skeletal muscle mass would be associated with higher pressure hyperalgesia and self-reported pain.
MethodsStudy designThis cross-sectional study was approved by the Universidade Estadual Paulista (UNESP), Presidente Prudente, SP, Brazil Ethics Committee (number: 1.484.129) and reported according to the STROBE and PFP-REPORT checklists.27,28 Informed consent was obtained from all participants.
ParticipantsOne hundred and fourteen people with PFP were recruited from the community between October 2018 and November 2019. Eligibility criteria were assessed by an experienced physical therapist (>7 years), based on a PFP consensus statement.2,8 To be included, participants had to be between 18 and 35 years old and present with anterior knee pain exacerbated by at least 2 of the following activities: running, walking, hopping, landing, squatting, stair negotiation, kneeling, or prolonged sitting. A pain duration of at least 3 months prior to study enrollment and a pain severity of at least 30 mm on a 100 mm visual analogue scale (VAS) in the previous month were also required. Participants were excluded if they presented a history of surgery on any lower limb joint; history of patellar subluxation; clinical evidence of meniscal injury,29 or ligament instability30; back, hip, ankle, or foot pain; recent or current physical therapy treatment for PFP (at least 6 months prior to data collection). The participants included were asked to refrain from any medications and avoid unaccustomed types of physical activity in the 7 days prior to data collection.
ProceduresParticipants attended a single testing session during which demographic data, self-reported measures of pain, bioelectrical impedance analysis, and measures of local, spread, and widespread pressure hyperalgesia (PPTs) were obtained. PPT in lower limb sites was assessed in the symptomatic leg or most symptomatic leg (in case of bilateral symptoms). The most symptomatic leg was self-reported by the participant at the data collection.
Demographics and self-reported measuresDemographic characteristics, including age, sex, height, and body mass were obtained, along with duration of symptoms (months) and presence of bilateral pain (yes/no). Height (cm) and body mass (kg) were measured to the nearest 0.1 cm and 0.1 kg, respectively, with the participant wearing light clothes and no shoes. BMI was calculated as body mass (kg) divided by the square of height (m2).
All participants were asked to rate their current knee pain intensity (prior to the PPT measurements) and their worst knee pain intensity during the last month, measured on a 0–100 VAS, with 0 indicating no pain and 100 indicating the worst pain possible.31
Bioelectrical impedance analysisBody fat and skeletal muscle mass were measured using a bioelectrical impedance analyzer (Omron HBF 514C; OMRON HEALTHCARE Co, Kyoto, Japan). A single test was conducted for each participant according to manufacturer instructions and all measurements were performed by the same trained rater. Participants stepped on the foot electrodes barefoot and held a pair of electrodes fixed on the display unit, with arms extended in front of their chest, until measurements were completed. The manufacturers’ valid and reliable equations32 were used to predict body fat and skeletal muscle mass (expressed as a percentage of total body mass). Participants were instructed to fast for at least 2 h prior to the measurements, to avoid alcohol and caffeine consumption during the previous 24 h, and not engage in vigorous exercise during the previous 12 h.
Reliability of the bioelectrical impedance analyzer is high to very high (intraclass correlation coefficient = 0.87 for women and = 0.96 for men). Validity based on the correlation with Air Displacement Plethysmography is also high (r = 0.89 for women and r = 0.94 for men).32
Pressure hyperalgesia (pressure pain thresholds)Pressure hyperalgesia was measured using a handheld digital algometer (PPTs) (Wagner FPXTM 25, Greenwich, CT, USA) with a probe size of 1cm2 and at a pressure rate of 0.50 kgf/s.10,11 PPTs were measured in a randomized order at the following body site: (1) local: center of patella of the most painful knee; (2) spread: ipsilateral tibialis anterior (muscle belly of tibialis anterior, 5 cm distal to the tibial tuberosity); and (3) widespread: contralateral upper limb (the lesser tubercle of the humerus).10,11,33 PPT measurements were conducted in the same laboratory for all participants, in a light, temperature, and sound-controlled room. Measurements were done with the participants lying in supine position on the examination table, and with the algometry tip placed perpendicular to the skin. Participants were instructed that an incremental pressure would be applied at three test sites (local, spread, and widespread) and that they should indicate when the sensation of pressure was perceived to become a sensation of pain by saying “stop.” At that moment, the algometer was immediately released, and the maximum applied force was read from the display of the algometer in kgf, which was defined as the PPT. Measurements were done twice at each site with a 30-s period between testing, and the average was used for analysis.10,11 All measurements were performed by the same rater.
A test-retest reliability was conducted in 20% of the sample (21 participants) on two separate days, with an interval of 2 to 7 days between assessments. We collected data on eight participants at the beginning, eight participants in the middle, and five participants at the end of the study, to avoid training bias. Intra-rater reliability for the measurements of pressure hyperalgesia was calculated using intraclass correlation coefficient (ICC2,k). We found moderate to good reliability for all measurements34: ICC2,2 (95% CI) = 0.80 (0.56, 0.91) for the center of patella; 0.62 (0.27, 0.82) for tibialis anterior; and 0.76 (0.31, 0.91) for the lesser tubercle of the humerus.
Statistical analysisSample size calculation was performed a priori according to Green recommendations.35 A minimum sample size of 104 + k (where k is the number of predictors) is suggested to test individual predictors within a regression model.35 Considering 4 predictors variables, a sample size of at least 108 (104 + 4) was required.
Data were analyzed using the PASW statistics software (Version 18; SPSS Inc., Chicago, IL). The significance level was set at 0.05 for all statistical analyses. Data were checked for normality and variance homogeneity using the Shapiro–Wilk test and Levene tests, respectively. When not normally distributed, the data were log-transformed before analyses. Descriptive statistics were used to describe participants characteristics, body fat, skeletal muscle mass, self-reported pain, and pressure hyperalgesia.
Partial correlation coefficients were used to quantify the association between independent variables (body fat, skeletal muscle mass, and BMI) with dependent variables (PPTs and current and previous month self-reported pain [VAS]), adjusting for covariates (sex, presence of bilateral pain, and duration of symptoms). All variables found to be significantly correlated (p < 0.05) were inserted into hierarchical regression models.
Separate hierarchical regression models were used to identify whether body fat, skeletal muscle mass, and BMI are significant predictors of the dependent variables (local, spread, and widespread pressure hyperalgesia and current and previous month self-reported pain) that presented significant correlations. To adjust the model for covariates, sex, bilateral symptoms, and duration of symptoms were entered into the hierarchical regression model first (model 1). Then, either body fat, skeletal muscle mass, or BMI was added into the model (model 2). This means that all changes in the results of the regression models, from the first to the second model, were due to the insertion of the independent variable (either body fat, skeletal muscle mass, or BMI). Fig. 1 shows in detail the experimental design of our study.
ResultsDescriptive characteristics of the participants including demographics, body fat, and skeletal muscle mass, pressure hyperalgesia, and self-reported pain are reported in Table 1.
Characteristics of the participants with PFP.
| Variables | All participants (n = 114) |
|---|---|
| Demographics | |
| Age (years) | 24.07 ± 4.75 |
| Body mass (kg) | 71.87 ± 15.13 |
| Height (m) | 1.68 ± 0.08 |
| BMI (kg/m²) | 25.22 ± 4.71 |
| Bilateral pain n (%) | 66 (58) |
| Sex (n;% females) | 71 (62) |
| Educational level, n (%) | |
| Under high-school | 1 (1) |
| Completed high-school | 11 (10) |
| Ongoing College/University | 63 (55) |
| Completed College/University | 39 (34) |
| Self-reported measures | |
| Current pain (VAS) | 17.59 ± 23.06 |
| Worst pain in the previous month (VAS) | 49.91 ± 21.82 |
| Duration of symptoms (months)† | 36 [12–96] |
| Bioelectric impedance analysis | |
| Body fat (%) | 31.26 ± 10.71 |
| Skeletal muscle mass (%) | 31.09 ± 7.20 |
| Pressure hyperalgesiaa | |
| Center of patella (kgf)† | 4.69 [3.44–5.74] |
| Ipsilateral tibialis anterior (kgf)† | 4.52 [3.51–5.87] |
| Contralateral upper limb (kgf)† | 3.01 [2.34–4.09] |
Data are presented as mean ± standard deviation unless otherwise stated. Abbreviations: BMI, body mass index; VAS, visual analogue scale.
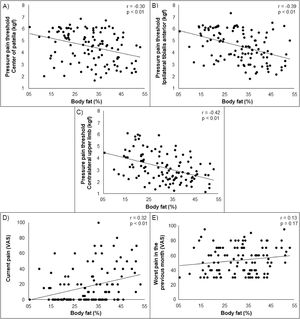
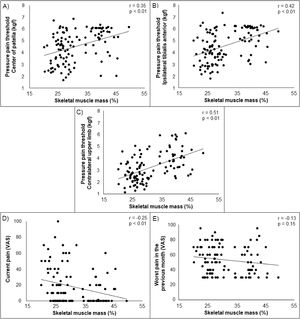
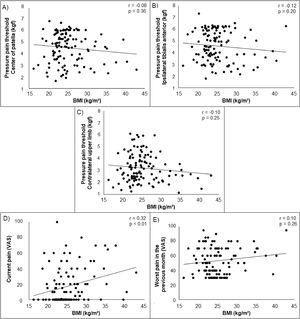
Higher body fat was associated with lower PPT on the center of patella, ipsilateral tibialis anterior, and contralateral upper limb (r = −0.42 to −0.30) and with higher current self-reported pain (r = 0.32) in young adults with PFP. Lower skeletal muscle mass was associated with lower PPT on the center of patella, ipsilateral tibialis anterior, and contralateral upper limb (r = 0.35 to 0.51) and with higher current self-reported pain (r = −0.25). Higher BMI was associated with higher current self-reported pain (r = 0.32). No significant associations were found between BMI and any measures of PPT. Additionally, no significant associations were found between body fat, skeletal muscle mass, and BMI with previous month self-reported pain (Supplementary material). Scatterplots illustrating the associations (non-adjusted) tested in our study are presented in Figs. 2, 3 and 4.
Body fat explained 9.1% of the variance in PPT on the center of patella (p = 0.001), 15.5% of the variance in PPT on the ipsilateral tibialis anterior (p < 0.001), 17.5% of the variance in PPT on the contralateral upper limb (p < 0.001), and 10.8% of the variance in current self-reported pain (p <0.001), after accounting for covariates (Table 2). Skeletal muscle mass explained 14.1% of the variance in PPT on the center of patella (p < 0.001), 17.6% of the variance in PPT on the ipsilateral tibialis anterior (p = < 0.001), 26.5% of the variance in PPT on the contralateral upper limb (p < 0.001), and 6.5% of the variance in current self-reported pain (p = 0.007), after accounting for covariates (Table 3). BMI explained 10.4% of the variance in current self-reported pain (p = 0.001), after accounting for covariates (Table 4).
Hierarchical linear regressiona for body fat, with measures of pressure hyperalgesia and self-reported pain.
| Dependent variable | Model | Independent variable | R² | ∆R² | F-change (sig. F-change) | B (95% CI) |
|---|---|---|---|---|---|---|
| Pressure hyperalgesiab | ||||||
| Center of patella (kgf)† | 1 | Covariatesa | 0.101 | |||
| 2 | Body fat | 0.192 | 0.091 | 11.193 (0.001) | −0.004 (−0.007, −0.002) | |
| Ipsilateral tibialis anterior (kgf)† | 1 | Covariatesa | 0.106 | |||
| 2 | Body fat | 0.261 | 0.155 | 20.632 (<0.001) | −0.005 (−0.007, 0.003) | |
| Contralateral upper limb (kgf)† | 1 | Covariatesa | 0.099 | |||
| 2 | Body fat | 0.274 | 0.175 | 23.678 (<0.001) | −0.006 (−0.009, −0.004) | |
| Self-reported pain (VAS) | ||||||
| Current pain | 1 | Covariatesa | 0.085 | |||
| 2 | Body fat | 0.193 | 0.108 | 13.358 (<0.001) | 0.67 (0.30, 1.04) |
Abbreviations: VAS, visual analogue scale.
Hierarchical linear regressiona for skeletal muscle mass, with measures of pressure hyperalgesia and self-reported pain.
| Dependent variable | Model | Independent variable | R² | ∆R² | F-change (sig. F-change) | B (95% CI) |
|---|---|---|---|---|---|---|
| Pressure hyperalgesiab | ||||||
| Center of patella (kgf)† | 1 | Covariatesa | 0.100 | |||
| 2 | Skeletal muscle mass | 0.241 | 0.141 | 18.360 (<0.001) | 0.071 (0.038, 0.104) | |
| Ipsilateral tibialis anterior (kgf)† | 1 | Covariatesa | 0.107 | |||
| 2 | Skeletal muscle mass | 0.283 | 0.176 | 24.100 (<0.001) | 0.078 (0.046, 0.109) | |
| Contralateral upper limb (kgf)† | 1 | Covariatesa | 0.100 | |||
| 2 | Skeletal muscle mass | 0.365 | 0.265 | 40.417 (<0.001) | 0.087 (0.060, 0.114) | |
| Self-reported pain (VAS) | ||||||
| Current pain | 1 | Covariatesa | 0.085 | |||
| 2 | Skeletal muscle mass | 0.150 | 0.065 | 7.617 (0.007) | −0.78 (−1.34, −0.22) |
Abbreviations: VAS, visual analogue scale.
Hierarchical linear regressiona between BMI and self-reported pain.
| Dependent variable | Model | Independent variable | R² | ∆R² | F-change (sig. F-change) | B (95% CI) |
|---|---|---|---|---|---|---|
| Self-reported pain (VAS) | ||||||
| Current pain | 1 | Covariatesa | 0.088 | |||
| 2 | BMI | 0.192 | 0.104 | 12.757 (0.001) | 1.48 (0.66 to 2.31) |
Abbreviations: BMI, body mass index; VAS, visual analogue scale.
Partial correlation coefficients (r)a for body fat, skeletal muscle mass, and BMI with measures of pressure hyperalgesia and self-reported pain.
| Variables | Body fat (%) r (p) | Skeletal muscle mass (%) r (p) | BMI (kg/m²) r (p) |
|---|---|---|---|
| Pressure hyperalgesiab | |||
| Center of patella (kgf)† | −0.30 (<0.01) | 0.35 (<0.01) | −0.08 (0.36) |
| Ipsilateral tibialis anterior (kgf)† | −0.39 (<0.01) | 0.42 (<0.01) | −0.12 (0.20) |
| Contralateral upper limb (kgf)† | −0.42 (<0.01) | 0.51 (<0.01) | −0.10 (0.25) |
| Self-reported pain (VAS) | |||
| Current pain | 0.32 (<0.01) | −0.25 (<0.01) | 0.32 (<0.01) |
| Worst pain in the previous month | 0.13 (0.17) | −0.13 (0.15) | 0.10 (0.26) |
Abbreviations: BMI, body mass index; VAS, visual analogue scale.
Our findings indicate that higher body fat and lower skeletal muscle mass were associated with higher local, spread, and widespread pressure hyperalgesia in young adults with PFP. Higher BMI was not associated with any measures of pressure hyperalgesia. Higher body fat and BMI and lower skeletal muscle mass were associated with higher current self-reported pain. Our findings provide important preliminary information regarding a link between body composition measures and pressure hyperalgesia in young adults with PFP, which might inform the design of future research, and management of PFP.
Previous studies evaluating the relationship between body fat and BMI with pressure hyperalgesia in injured and non-injured populations presented conflicting findings.20,25,36 Tashani et al.25 and Zhang et al.36 reported similar findings to those observed in our study, with higher body fat and BMI associated with lower PPT in a non-injured mixed-sex and male only population, respectively. Higher BMI was also associated with lower PPTs in people with low back pain.20 However, when comparing non-injured people with different ranges of BMI (i.e., obese vs. non-obese), Price et al.37 did not find any difference in PPTs. This inconsistency may be due to differences in study methodologies, including injured vs non-injured populations and testing sites with different accumulations of subcutaneous fat. Collectively, this may reinforce that the relationship between hyperalgesia and obesity is specific to some populations and linked to pathology.
Different mechanisms may explain the findings reported in our study. One of them is the systemic effect associated with increased body fat, including the production of pro-inflammatory cytokines by adipose tissue resulting in sensitization of peripheral nociceptors and central nociceptive transmission pathways.19,22 Another is that elevated pro-inflammatory cytokines have been associated with loss of skeletal muscle mass,24 while higher skeletal muscle mass has been associated with greater conditioned pain modulation,38 a measure of endogenous pain inhibition. Lower skeletal muscle mass may therefore be resulting in a more persistent inflammatory state, once again leading to more prolonged peripheral nociceptor activation and subsequent sensitization. We found that skeletal muscle mass was the best predictor of hyperalgesia, explaining 14.1%, 17.6%, and 26.5% of the variance in local, spread, and widespread hyperalgesia, respectively. While body fat explained 9.1%, 15.5%, and 17.5%, respectively. A possible explanation for these findings is that people who have higher levels of skeletal muscle mass are more physically active.39 Previous studies have found associations between higher levels of physical activity and higher levels of pain and hyperalgesia.11,40
Contrary to our hypothesis, BMI was not associated with any measures of pressure hyperalgesia. Theoretically, increases in body mass, and consequently in BMI, could heightens the load on an already irritated patellofemoral joint,41 leading to sustained activation of nociceptors and sensitization, resulting in hyperalgesia.10 However, the fact that the body fat and skeletal muscle mass, but not BMI, were associated with pressure hyperalgesia reinforces that the mechanism underpinning pain sensitization may not simply be via loading of the joint, but perhaps via the systemic effect occurring as a consequence of increased adiposity (i.e., higher body fat) and reduced muscle mass (i.e., lower skeletal muscle mass). BMI does not include specific components of body composition (i.e., body fat and skeletal muscle mass); therefore, advanced methods to assess body composition may be more appropriate for PFP assessment.
Body fat, skeletal muscle mass, and BMI were associated with current self-reported pain, explaining 10.8%, 6.5%, and 10.4% of its variance, respectively. These findings add to the recent evidence indicating the potential impact of body composition measures on PFP.16 However, no significant associations were found between body fat, skeletal muscle mass, and BMI with worst previous month self-reported pain. Interestingly, from the 114 people assessed in our study, 53 presented current VAS pain equal to zero; of those, 79% were normal weight based on their BMI. It is possible that overweight and obese people with PFP have a more constant pain characteristic due to the sustained nociceptive stimulus as a consequence of the systemic effects of adiposity and decreased skeletal muscle mass. In turn, people with PFP who are of normal weight may have a more intermittent pain characteristic, that is usually associated with the clinical presentation of people with PFP.40 Therefore, current and worst pain in the previous month may reflect different pain characteristics of people with PFP.
Pain is complex and multifactorial. Body composition measures (body fat, skeletal muscle mass, and BMI) alone are unlikely to explain all variances in symptomatology observed within this patient population.42 The assessing and treating clinician should continue to maintain a balanced approach to considering the psychosocial (e.g. kinesiophobia, pain catastrophizing, anxiety),43–47 biomechanical, activity volume, and structure of the individual presenting with PFP.48
Clinical implications and future researchGiven our findings that body fat and skeletal muscle mass are related to pressure hyperalgesia, and that body fat, skeletal muscle mass, and BMI are related with self-reported pain in young adults with PFP, these factors should be investigated for their potential to predict treatment response and long-term prognosis. The possible systemic effects associated with being overweight and obese remain unexplored. To date there are no data on the levels of pro-inflammatory cytokines in people with PFP. Further studies are needed to explore whether people who are overweight or obese and have PFP have a different inflammatory profile compared to people with PFP who are of a normal weight. It is possible that treatments targeting body fat reduction and increases in skeletal muscle mass may directly or indirectly influence the pressure hyperalgesia in those with PFP, having a positive impact on the poor long-term outcomes associated with the condition.3,49 A recent study reported a link between weight loss and improvements in pressure hyperalgesia in people with knee pain who were obese and underwent bariatric surgery.50 Additionally, reductions in self-reported knee pain following weight loss seems to be mediated by improvements in pressure hyperalgesia.51
These findings may have an impact on the physical therapists practice by further informing the assessment and management of people with PFP. When possible, physical therapists should include the assessment of body composition measures. Although diet prescription may be outside their scope of practice, physical therapists have an important role in educating patients about the impact of overweight and obesity for their condition. Prescribing and progressing exercises that targeted strength, power, and endurance of the hip and knee muscles, encouraging patients to adequately increase their levels of physical activity and give advice on having a healthy lifestyle represents interventions that a physical therapist is well placed to prescribe.52–55 To date there is no evidence-based recommendations regarding weight management for the treatment of PFP.26 Future clinical trials are required to determine whether reducing body fat and BMI and increasing skeletal muscle mass mediates improvements in pain experience of people with PFP. Regardless of the association between hyperalgesia and body composition measures being specific or not to PFP, overweight and obesity are related to many comorbidities, including knee osteoarthritis, cardiovascular disease, and diabetes.56,57 This risk factor for the development of chronic conditions should be considered during patient management.
LimitationsOur study has a cross-sectional design; thus, causality cannot be inferred. Our sample has a lower proportion (13%) of individuals in the obese BMI category,58 which may limit the study power to detect associations between BMI and pressure hyperalgesia. However, this reflects the prevalence of obesity in the general population with PFP.1 Body fat and skeletal muscle mass were assessed using a bioelectrical impedance analyzer and only one measure was taken for all participants; the average of two or three measures could have led to slightly different results. The magnitude of R² values were generally low; although body fat, skeletal muscle mass and BMI, were not included in the same models due to collinearity, these three measures are likely to be related and a significant overlap in their explained variance may have occurred. It is possible that the subcutaneous adipose thickness of the measurement's sites influences the transmission of externally applied pressure, which in turn may affect the pressure-induced pain sensation. To control that the adipose thickness would not influence our PPT measurements, the placement of the algometer was done in sites with different accumulations of subcutaneous fat (i.e., patellar and humerus bone, and tibialis anterior soft tissue); however, this approach may explain the lesser explained variance found at the patella, where there is lower accumulation of subcutaneous fat, compared to the other assessed sites. We only used pressure stimuli to assess hyperalgesia. Further investigation of the relationship between body compositions measures (i.e., body fat, skeletal muscle mass and BMI) with an expanded quantitative sensory testing protocol, may contribute to a broader understanding of the sensitization processes occurring in PFP.
ConclusionHigher body fat and lower skeletal muscle mass, but not BMI, were associated with local, spread, and widespread pressure hyperalgesia in young adults with PFP. Higher body fat and BMI, and lower skeletal muscle mass were associated with higher self-reported pain. Collectively, these findings suggest that young adults with PFP might benefit from more detailed assessment of body composition in the formulation and prescription of an individualized management plan.
The authors would like to acknowledge the Sao Paulo Research Foundation - FAPESP (scholarship n° 2018/17106-0). The financial sponsors played no role in the design, execution, analysis, and interpretation of data or writing of the study.
Received: 22 June 2021; Revised: 11 February 2022; Accepted: 22 June 2022, Original