Physical exercise is widely recognized for reducing neuropathic pain. However, the interaction between the immune and opioidergic systems in supraspinal structures is still not fully understood.
ObjectiveTo evaluate the impact of opioid receptor blockade on the effects of low-intensity exercise on the sensory, cognitive, and emotional aspects of neuropathic pain after sciatic nerve injury.
MethodsMale Swiss mice (2 months old) were submitted to sciatic nerve crush and divided into sedentary or exercised groups. The exercised groups performed treadmill running for two weeks, with or without naloxone pre-treatment to block opioid receptors. Sensory responses were assessed using the von Frey test, while cognitive and emotional-like behaviors were evaluated through the Mechanical Conflict-Avoidance System (MCAS) and open field test, respectively. Cytokine levels (IL-4, IL-10) and brain-derived neurotrophic factor (BDNF) were quantified in the brainstem and prefrontal cortex by ELISA.
ResultsExercise reduced mechanical hypersensitivity and improved performance in cognitive and exploratory tasks. These effects were prevented by naloxone administration. Exercise also increased IL-4, IL-10, and BDNF levels in supraspinal regions, while naloxone reversed these changes, indicating the involvement of μ-opioid receptors in exercise-induced immunomodulation.
ConclusionLow-intensity exercise promotes analgesia and neuroimmune regulation in neuropathic pain through supraspinal μ-opioid receptor activation. The blockade of these receptors abolishes the beneficial effects of exercise, reinforcing the interaction between opioidergic and immune systems in pain modulation.
Neuropathic pain, often resulting from injuries to the peripheral somatosensory system, significantly impairs quality of life, affecting approximately 6.9 % to 10 % of the global population.1 One-third of those affected experience difficulties with daily activities,2 and treatment costs in the U.S. reach around $5000 annually per patient, with indirect expenses up to $27,000.3
Neuroimmune interactions are fundamental to neuropathic pain, contributing to nociceptor sensitization and both peripheral and central sensitization following peripheral nervous system (PNS) injury.4 Immune cells like macrophages and T lymphocytes migrate to the injury site, activating neurons in the spinal cord and brain, a process known as central sensitization.5 Within this context, endogenous opioids (β-endorphins) act on opioid receptors to relieve pain. Naloxone, an opioid receptor antagonist, blocks these effects, underscoring the opioid system’s role in pain regulation.6,7
Exercise, recognized for broad health benefits, enhances opioid system function, increasing endogenous opioids such as endorphins and supporting pain modulation.8 It also stimulates Th2 cytokines, particularly IL-4 and IL-10, which reduce neuroinflammation.9 Moreover, exercise influences BDNF, vital for neuronal plasticity, suggesting potential benefits for functional recovery after PNS injuries.10–12 In this study, IL-4 and IL-10 are considered for their anti-inflammatory properties, while BDNF may support neuronal repair.
Although exercise reduces the risk of chronic pain, the interplay between β-endorphins, exercise, and Th2 cytokines requires further investigation.13–15 Research remains limited on how these neuroimmune interactions affect analgesic outcomes, particularly regarding the roles of IL-4 and BDNF in opioid receptor modulation.16–19
Based on previous findings, we hypothesize that low-intensity physical exercise induces analgesic and anti-inflammatory effects after peripheral nerve injury, which are mediated by endogenous opioid mechanisms involving the modulation of IL-4, IL-10, and BDNF levels in the prefrontal cortex (PFC) and brainstem. Thus, this study evaluates the impact of low-intensity exercise on neuroimmune interactions, focusing on the opioid system, BDNF, and Th2 cytokines and its effects on pain and neuroinflammation following PNS injury.
MethodsAnimalsThis research was approved by the Ethics Committee on Animal Use (CEUA) of University of Southern Santa Catarina (UNISUL) (Brazil) (protocol 21.004.4.01.IV) and was conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No 80–23).
The experiments were carried out using male Swiss mice (Mus musculus). The mean weight at baseline was 39.5 ± 3.1 g, and the age was 60 ± 4 days. The animals were obtained from the vivarium of the Federal University of Santa Catarina (UFSC), Brazil, and housed at the Experimental Neuroscience Laboratory (LaNEx), UNISUL, in a room maintained at 22 ± 2 °C, on a 12-hour light/dark cycle (lights on at 6:00 a.m.), with ad libitum access to food and water.
Sample size determination was performed through statistical calculations, using a sample without replacement. The formula used was: n = ((z_alpha + z_beta) * s) / (sigma)².20 Applying the values to the formula, we determined n = ((1.96 + 1.28) * 35) / (40)² = 8 animals per group for each outcome studied. A blinded experimenter performed simple randomization by drawing lots to assign the animals to the experimental groups. The animals were then distributed into five experimental groups: (1) Sham/Saline/Non-Exercised (n = 8); (2) Sham/Saline/Exercised (n = 8); (3) Nerve Injury/Saline/Non-Exercised (n = 8); (4) Nerve Injury/Saline/Exercised (n = 8); and (5) Nerve Injury/Naloxone/Exercised (n = 8). Additionally, (6) Nerve Injury/Morphine/Non-Exercised (n = 8), and (7) Nerve Injury/Naloxone/Morphine/Non-Exercised (n = 8), with these two groups highlighted in the Online material, totaling 56 animals. The animals were then randomly housed in polypropylene cages with stainless steel grids and bedding of wood shavings, with a maximum of 20 animals per cage.
Study designThe animals were acclimated to treadmill training for 6 days before sciatic nerve surgery. All animals were acclimated to treadmill training, including both exercised and non-exercised groups. The animals in the exercise group underwent training on a moving treadmill, while the non-exercised group were maintained on a stationary treadmill. All groups were evaluated 24 h before the nerve injury to record baseline data on sensitivity to mechanical stimuli (von Frey test) and pain-related cognitive behavior (MCAS). After the surgery, a treadmill exercise protocol was initiated for two weeks in the following groups: Sham/Saline/Exercised, Nerve Injury/Saline/Exercised, and Nerve Injury/Naloxone/Exercised. The von Frey test was performed daily for two weeks, the open field test on the 13th day, and the MCAS on the 14th day after the injury. Twenty-four hours after the last exercise session, on the 15th day, the animals were euthanized, and samples from the PFC and brainstem were collected for ELISA and Western blotting. All evaluations were performed by researchers blinded to the animals' allocation and the treatment received, following ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.21
Induction of neuropathic pain by crushing the sciatic nerveThe animals were pre-anesthetized with an intraperitoneal injection of xylazine (10 mg/kg) and ketamine (80 mg/kg). After verifying the animals' level of consciousness, the area to be operated on was shaved, and the animals were maintained under anesthesia with inhaled isoflurane (1–2 % in 100 % oxygen). A curvilinear incision was made on the right thigh, and the sciatic nerve was exposed after separating the semitendinosus and rectus femoris muscles. In the Sham groups, the animals underwent the same surgical procedure, but without crushing the sciatic nerve. In the other groups, the right sciatic nerve was compressed for 30 s using a non-serrated hemostat, applying pressure until the first stage of the clamp, approximately 10 mm above the nerve trifurcation. The skin was then repaired with two sutures using absorbable thread, and the site was disinfected. After surgery, the animals were kept near a heat source until they resumed walking and then returned to the vivarium. The surgery was always performed by the same researcher to ensure consistency.12
To refine the experimental model and ensure animal welfare, a scale was used to monitor signs of stress and pain. Animals showing severe pain, self-mutilation, vocalization without stimulation, or vocalization with stimulation were excluded, with humane euthanasia applied as per National Council for the Control of Animal Experimentation – Brazil (CONCEA) guidelines. Exclusion criteria included apathy, signs of systemic infection, seizures, motor abnormalities, inability to move, immobility, inability to eat or drink, 20 % weight loss, or dehydration for >48 h.
Low-intensity aerobic exercise protocolThe animals were habituated to a treadmill adapted for mice for six days before sciatic nerve surgery, running for 5 min per day at a speed of 10 m/min to minimize the effects of stress in an unfamiliar environment. The exercise was performed on a treadmill with 12 individual acrylic lanes (25 × 10 × 9.5 cm; EMBREEX®, Curitiba, PR, Brazil). The protocol consisted of running on the treadmill for 30 min per day at a constant speed of 10 m/min, with no incline, five days per week, for a period of two weeks. The treadmill speed corresponding to 75 % of the maximum lactate steady-state (MLSS) was adopted from previously validated protocols in Swiss mice of similar age and weight, who identified the MLSS at 13.3 m/min.12 No direct measurement of lactate levels was performed in this study; instead, the intensity was inferred from the literature. It is worth noting that the use of an exercise intensity equivalent to 75 % of the MLSS is common practice in experimental models of low-intensity aerobic training and reflects a metabolically stable zone, characterized by predominant oxidative metabolism and low lactate production.22
No electrical, mechanical, or manual aversive stimuli were used during treadmill training. Animals spontaneously maintained the programmed running speed throughout the sessions. Physical exercise treatment started on the third day after nerve injury. The animals from the untrained groups (Sham/Saline/Non-Exercised and Nerve Injury/Saline/Non-Exercised) were merely handled and placed on the stationary treadmill for the same amount of time.
Investigation of the opioid receptor in the effect of physical exerciseTo study the role of opioid receptors in physical exercise-induced analgesia, mice were pre-treated daily for two weeks with naloxone (5 mg/kg) or saline (10 ml/kg) before each physical exercise session. Morphine (5 mg/kg) was used as a control to assess naloxone's ability to counteract its analgesic effect. After 30 min, physical exercise sessions followed the described protocol, and mechanical hyperalgesia was assessed. Morphine-treated animals did not perform physical exercise but were placed on the treadmill for the same duration.13,14
All physical exercise sessions were conducted in the morning (between 8:00 and 11:00 AM) to minimize circadian influences. The study was conducted daily for two weeks post-nerve injury. Experimental groups included: (1) Sham/Saline/Non-Exercised (n = 8); (2) Sham/Saline/Exercised (n = 8); (3) Nerve Injury/Saline/Non-Exercised (n = 8); (4) Nerve Injury/Saline/Exercised (n = 8); (5) Nerve Injury/Naloxone/Exercised (n = 8); (6) Nerve Injury/Morphine (n = 8); and (7) Nerve Injury/Naloxone/Morphine (n = 8).
Assessment of mechanical hyperalgesiaMechanical hyperalgesia was assessed using a von Frey monofilament with a 0.6 g load (VFH, Stoelting Co., Wood Dale, IL, USA). The response frequency (percentage) of withdrawal from the right hind paw was recorded over 10 filament applications.23 Animals were placed in an acrylic box (9 × 7 × 11 cm) on a platform with a 6 mm wire mesh, allowing access to the paw's plantar surface. The filament was applied perpendicularly for 5 s, and a positive response was recorded when the animal completely withdrew its paw from the mesh.
Mechanical conflict-avoidance systemThe MCAS assesses the pain-related cognitive component in a sciatic nerve crush model. The test uses a device with three acrylic chambers: two red and transparent and one black (Alumecril, São José, SC, Brazil). The first chamber, measuring 12.5 cm³, is illuminated by an LED, creating an aversive stimulus for the animal to move towards the darker chambers, and is separated by a movable barrier. The second chamber, 27 cm long, connects chambers 1 and 3. The third chamber, completely dark and also 12.5 cm³, is at the opposite end. The floor of chamber 2 consists of smooth acrylic or steel with 0.5 mm diameter probes, set at heights of 2 mm or 5 mm, providing a mechanical nociceptive stimulus. Animals were placed in chamber 1 with the barrier closed. After 15 s, the LED was turned on, and the barrier was removed 20 s later. The time taken for animals to leave chamber 1, cross the platform with probes, and enter chamber 3 was recorded as latency.24
Open field testThe open field test (OFT) was used to evaluate spontaneous locomotor activity and thigmotaxis (anxiety-like behavior), which may provide insights into the affective dimension of chronic pain. The test used a 40 × 40 × 40 cm wooden arena (Alumecril, São José, SC, Brazil). Each animal was placed in the center of the arena and allowed to explore freely for 5 min. Exploratory behaviors were recorded, including total distance traveled, number of entries into the center, and time spent in the center, and were analyzed using ANY-maze® software (ANY-maze, Stoelting Co., Wood Dale, IL, USA). The apparatus was cleaned with a 10 % ethanol solution between tests.25
Biochemical assaysApproximately 24 h after the final behavioral assessment, on the 15th day following sciatic nerve injury, the animals were anesthetized with inhaled isoflurane and euthanized by decapitation. The brainstem and spinal cord were collected for ELISA and Western blotting assays, frozen in liquid nitrogen, and stored at −80° C until analysis.
Enzyme-linked immunosorbent assayFor IL-4, IL-10, and BDNF assessment, PFC and brainstem samples were homogenized in PBS with Tween 20, benzethonium chloride, EDTA, NaCl, aprotinin A, PMSF, and BSA. The homogenates were centrifuged at 3000 × g for 10 min at 4 °C, and the supernatant was used for the assay. Interleukins and BDNF levels were measured using mouse ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Protein content was assessed with the Bradford method,26 and concentrations were expressed as pg cytokine/mg protein. Results were read with a plate reader (Perlong DNM-9602, Nanjing Perlove Medical Equipment Co, Nanjing, China).
Western blottingThe Western blotting assay quantified µ-opioid receptors (MOR). Samples were homogenized and incubated in RIPA lysis buffer with 1 % protease inhibitor cocktail, then centrifuged at 6000 × g for 20 min (4 °C). Protein content was measured using the Bradford method.26 Protein aliquots (20 µg) were boiled in Laemmli buffer, subjected to 10 % SDS-PAGE, and transferred to a PVDF membrane. The membrane was blocked and incubated with primary antibodies: Rabbit anti-µ opioid receptor (1:5000, AB1580-I, Sigma Aldrich, Darmstadt, Germany) and Anti-β-Actin-Peroxidase (1:45,000; A3854, Sigma-Aldrich, St. Louis, MO, USA). After washing, secondary antibodies conjugated to peroxidase were applied. Detection was done using a chemiluminescence kit (ECL) and iBright Imaging Systems (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA). Bands were analyzed by densitometry, normalized to β-actin, and expressed as arbitrary units.
Statistical analysesResults were analyzed using GraphPad Prism 8.0. Data distribution was assessed with the Shapiro-Wilk test, and parametric results were presented as mean ± standard deviation (SD). Comparisons were made using one-way or two-way ANOVA with Tukey's post-hoc test. Tissue samples were analyzed for BDNF, IL-10, and IL-4 concentrations, and immunostaining of µ-opioid receptors. Pearson's correlation coefficient was used to evaluate correlations, with values interpreted as strong (0.70–1.0), moderate (0.30–0.70), or weak (0–0.30) positive, and strong (−0.70 to −1.0), moderate (−0.30 to −0.70), or weak (0 to −0.30) negative. P-values ≤ 0.05 were considered statistically significant.
ResultsThe sciatic nerve crush induced marked mechanical hyperalgesia in the Nerve Injury/Non-Exercised group from the 11th to the 14th day post-injury, with significantly lower withdrawal thresholds compared to the Sham/Non-Exercised group (mean difference [MD]= –26.25, 95 % CI: –46.16, –6.34 on the 11th and 12th days; and MD= –52.50, 95 % CI: –72.41, –32.59 on the 13th and 14th days; p < 0.05; Fig. 1A). Low-intensity treadmill exercise significantly reduced mechanical hyperalgesia in the Nerve Injury/Exercised group compared to the Nerve Injury/Non-Exercised group, with higher withdrawal thresholds observed on the 9th (MD = 20.00, 95 % CI: 0.65, 39.35), 12th (MD=23.06, 95 % CI: 3.71, 42.41), 13th (MD=46.67, 95 % CI: 27.32, 66.02), and 14th days post-injury (MD=46.67, 95 % CI: 27.32, 66.02; p < 0.05; Fig. 1A), demonstrating the analgesic efficacy of exercise. Naloxone, an opioid receptor antagonist, blocked the analgesic effect of exercise from the 12th to the 14th day post-injury, as demonstrated by lower withdrawal thresholds in the Nerve Injury/Naloxone/Exercised group compared to the Nerve Injury/Saline/Exercised group (MD = –19.56, 95 % CI: –37.85, –1.26 on the 12th day; MD = –33.67, 95 % CI: –51.96, –15.37 on the 13th day; and MD= –34.67, 95 % CI: –52.96, –16.37 on the 14th day; p < 0.05; Fig. 1A), indicating that the beneficial effects of exercise depend on opioid receptor activation.
Assessment of the effect of low-intensity physical exercise on mechanical hyperalgesia and pain-related cognitive capacity. Mechanical hyperalgesia assessments using the von Frey test (0.6 g) were performed before nerve injury (Baseline) and from the 3rd to the 14th day after injury, 0.5 h after the exercise session (A). Assessments of the cognitive component related to neuropathic pain using the Mechanical Conflict-Avoidance System were conducted at baseline (B) and on the 14th day after sciatic nerve crush (C), 2 h after treadmill exercise sessions. The assessments were carried out with the apparatus track without nociceptive probes (0 mm) and with nociceptive probes of 2 mm and 5 mm. All assessments were performed in the Sham/Saline/Non-Exercised (n = 8), Sham/Saline/Exercised (n = 8), Nerve injury/Saline/Non-Exercised (n = 8), Nerve injury/Saline/Exercised (n = 8), and Nerve injury/Naloxone/Exercised (n = 8) groups. Values represent the mean ± SD, with groups compared statistically using a two-way ANOVA followed by Tukey's post-hoc test. ## P < 0.05 when comparing Sham/Saline/Non-Exercised vs. Nerve injury/Saline/Non-Exercised groups; * p < 0.05 when compared to the Nerve injury/Saline/Non-Exercised group; & p < 0.05 when compared to the Nerve injury/Saline/Exercised group.
Baseline assessments in the MCAS showed no significant differences between groups prior to sciatic nerve injury, including between Nerve Injury/Non-Exercised and Sham/Non-Exercised animals, or between Nerve Injury/Exercised and Nerve Injury/Non-Exercised groups (Fig. 1B). On the 14th day post-injury, animals in the Nerve Injury/Saline/Non-Exercised group exhibited greater escape latency compared to Sham/Saline/Non-Exercised controls (MD = –102.1, 95 % CI: –150.7, –53.54; p < 0.05; Fig. 1C), confirming the development of pain-related cognitive impairment. Low-intensity exercise significantly reduced this latency in the Nerve Injury/Saline/Exercised group compared to the Nerve Injury/Saline/Non-Exercised group (MD = 93.91, 95 % CI: 52.85, 135.0; p < 0.05), indicating an improvement in pain-related behavior. However, naloxone administration abolished this effect, as the Nerve Injury/Naloxone/Exercised group showed increased latency compared to the Nerve Injury/Saline/Exercised group (MD = –54.84, 95 % CI: –94.82, –14.86; p < 0.05; Fig. 1C), demonstrating that the beneficial behavioral effects of exercise depend on opioid receptor activation.
Morphine, used as a positive control, effectively alleviated mechanical hyperalgesia, and this effect was prevented by naloxone pretreatment. Morphine also improved pain-related behavior in the MCAS test, an effect that was not influenced by naloxone pretreatment. Detailed results are presented in the Online material (Fig. S1A–B).
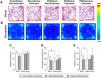
On the 13th day after sciatic nerve injury, the animals were subjected to the open field test (Fig. 2). Figs. 2A and 2B illustrate the paths taken by the animals and their occupancy within the apparatus, respectively. ANOVA revealed no significant differences in total distance traveled (Fig. 2C), suggesting no motor impairments that could interfere with behavioral analysis. Animals in the Nerve Injury/Saline/Non-Exercised group showed fewer entries into the central area and spent less time in that region compared to the Sham/Saline/Non-Exercised controls (MD = 6.85, 95 % CI: 2.04, 11.65 for the number of center entries; and MD = 15.85, 95 % CI: 1.80, 29.90 for time spent in the center; p < 0.05; Fig. 2D–E). No significant differences were observed between the Nerve Injury/Saline/Non-Exercised and Nerve Injury/Saline/Exercised groups, nor between the Nerve Injury/Saline/Exercised and Nerve Injury/Naloxone/Exercised groups, indicating that naloxone did not influence the effects of exercise on exploratory behavior (Fig. 2E).
Effect of low-intensity treadmill exercise following sciatic nerve crush on the open field test. Illustrative images of the routes taken by the animals (A) and occupancy in the open field apparatus (B). Number of entries into the center of the apparatus (D). Total time spent in the central region of the open field apparatus (E). Values represent the mean ± SD of animals from the groups: Sham/Saline/Non-Exercised (n = 8), Sham/Saline/Exercised (n = 8), Nerve injury/Saline/Non-Exercised (n = 8), Nerve injury/Saline/Exercised (n = 8), and Nerve injury/Naloxone/Exercised (n = 8), statistically compared using a two-way ANOVA followed by Tukey's post-hoc test. # p < 0.05 when comparing Sham/Saline/Non-Exercised vs. Nerve injury/Saline/Non-Exercised groups.
On the 15th day post-injury, sciatic nerve crush significantly increased IL-4, IL-10, and BDNF concentrations in the brainstem of the Nerve Injury/Saline/Non-Exercised group compared to the Sham/Saline/Non-Exercised group (MD = –0.79, 95 % CI: –1.45, –0.12 for IL-4; MD= –4.47, 95 % CI: –8.37, –0.58 for IL-10; and MD = –10.90, 95 % CI: –20.94, –0.86 for BDNF; p < 0.05; Fig. 3A–C). Physical exercise for two weeks reduced BDNF levels in the Nerve Injury/Saline/Exercised group compared to the Nerve Injury/Saline/Non-Exercised group (MD = 15.01, 95 % CI: 5.44, 24.58; p < 0.05; Fig. 3C). Naloxone administration decreased IL-4 and IL-10 levels and further reduced BDNF concentrations in the Nerve Injury/Naloxone/Exercised group compared to the Nerve Injury/Saline/Exercised group (MD = 0.74, 95 % CI: 0.08, 1.40 for IL-4; MD = 3.89, 95 % CI: 0.00, 7.79 for IL-10; and MD = 10.10, 95 % CI: 0.24, 19.96 for BDNF; p < 0.05; Fig. 3A–C).
Effect of low-intensity physical exercise 15 days after sciatic nerve crush injury on the concentrations of anti-inflammatory cytokines IL-4 (A and D) and IL-10 (B and E), and brain-derived neurotrophic factor (BDNF) (C and F) in the brainstem and prefrontal cortex (PFC). Data are expressed as mean ± SD and statistically compared using a two-way ANOVA followed by Tukey's post-hoc test in the following experimental groups: Sham/Saline/Non-Exercised (n = 6), Sham/Saline/Exercised (n = 6), Nerve injury/Saline/Non-Exercised (n = 6), Nerve injury/Saline/Exercised (n = 6), and Nerve injury/Naloxone/Exercised (n = 6). # p < 0.05 when comparing Sham/Saline/Non-Exercised vs. Nerve injury/Saline/Non-Exercised groups; * p < 0.05 when compared to the Nerve injury/Saline/Non-Exercised group; & p < 0.05 when compared to the Nerve injury/Saline/Exercised group.
In the PFC, sciatic nerve injury increased BDNF levels compared to the Sham/Saline/Non-Exercised group (MD = –10.90, 95 % CI: –20.76, –1.04; p < 0.05; Fig. 3F), while IL-4 and IL-10 remained unaffected. Exercise reduced IL-4, IL-10, and BDNF concentrations in the Nerve Injury/Saline/Exercised group compared to the Nerve Injury/Saline/Non-Exercised group (MD = 1.44, 95 % CI: 0.26, 2.61 for IL-4; MD = 8.27, 95 % CI: 3.08, 13.47 for IL-10; and MD = 21.82, 95 % CI: 4.69, 38.95 for BDNF; p < 0.05; Fig. 3D–F). Naloxone pretreatment before exercise prevented the reduction in IL-4 and BDNF concentrations in the PFC (MD = –1.22, 95 % CI: –2.39, –0.04] for IL-4; MD = –16.39, 95 % CI: –32.13, –0.65 for BDNF; p < 0.05; Fig. 3D, 3F).
We also investigated the effect of physical exercise on µ-opioid receptors in the brainstem and PFC on the 15th day after injury (Fig. 4A and B). ANOVA showed no significant differences between the groups with sciatic nerve crush injury, low-intensity physical exercise, and pre-administration of naloxone during physical exercise in the evaluated structures.
Effect of low-intensity physical exercise 15 days after sciatic nerve crush injury on µ-opioid receptor immunostaining in the brainstem (A) and prefrontal cortex (PFC) (B). Data are expressed as mean ± SD and statistically compared using a two-way ANOVA followed by Tukey's post-hoc test in the following experimental groups: Sham/Saline/Non-Exercised (n = 8), Sham/Saline/Exercised (n = 8), Nerve injury/Saline/Non-Exercised (n = 8), Nerve injury/Saline/Exercised (n = 8), and Nerve injury/Naloxone/Exercised (n = 8).
The concentrations of IL-4, IL-10, and BDNF were correlated with MOR content in the brainstem and PFC using Pearson's correlation test. No significant correlations were found in the brainstem: IL-4 (r = −0.1783; p = 0.291), IL-10 (r = −0.1217; p = 0.466), and BDNF (r = −0.2727; p = 0.097). Similarly, no significant correlations were found in the PFC: IL-4 (r = 0.6679; p = 0.690), IL-10 (r = 0.0081; p = 0.961), and BDNF (r = 0.0138; p = 0.934) (Fig. 5).
DiscussionOur main findings confirm the role of the µ-opioid receptor in the anti-hyperalgesic effects of low-intensity exercise, and highlight, for the first time to our knowledge, its involvement in reducing escape latency to an aversive light stimulus in the MCAS test suggesting an opioid-mediated modulation of supraspinal components of neuropathic pain. Exercise reduced IL-4, IL-10, and BDNF levels in the PFC, and BDNF in the brainstem. Naloxone pre-treatment prevented the exercise-induced reductions of IL-4 and BDNF in the PFC, but independently reduced IL-4 and IL-10 levels, and further decreased BDNF in the brainstem.
The sciatic nerve crush model induces behaviors related to neuropathic pain, such as exaggerated responses to mechanical stimuli (hyperalgesia).27 The apparent reduction in hyperalgesia on day 3 in the nerve injury groups likely reflects motor impairment from the sciatic nerve crush, which limited the animals’ ability to lift the paw during von Frey testing. This aligns with evidence that axonotmesis and Wallerian degeneration cause transient loss of motor and sensory conduction,28,29 leading to flaccid paralysis and diminished withdrawal reflexes. As a result, mechanical thresholds may appear falsely elevated due to sensorimotor dissociation, reinforcing the need for cautious interpretation of behavioral tests in the acute post-injury phase. Hyperalgesia developed from day 11 onward, consistent with previous reports on the onset of this condition. Kim et al.17 reported tactile hypersensitivity following partial, but not complete, sciatic nerve crush, supporting our findings regarding sensory changes induced by this model.12,30
Low-intensity exercise promoted an anti-hyperalgesic effect at the end of the protocol, which is consistent with studies describing exercise-induced analgesia in rodents subjected to sciatic nerve crush.11–13,31,32 Naloxone administration blocked this analgesic effect, reinforcing the role of endogenous opioids, already well-documented in the literature. Our results confirm that low-intensity exercise promotes opioid-mediated analgesia similarly to higher-intensity protocols.14,17,33
The von Frey test evaluates mechanical cutaneous sensitivity mediated by the spinal cord,34 while the MCAS test addresses cognitive and motivational aspects of pain.35 On day 14 after injury, animals showed increased latency to cross the apparatus with the nociceptive probe, indicating heightened pain sensitivity, consistent with other neuropathic models.23,36–38 Exercise reduced this latency, suggesting that low-intensity exercise may also modulate the cognitive component of neuropathic pain. Previous studies using this same model and protocol have demonstrated behavioral improvements in pain-related responses.13 Additionally, exercise reduced the latency to cross from the light to dark compartment in the MCAS test, as described by Chhaya et al.37 in spinal cord injury models. Naloxone pre-administration prevented the latency reduction, suggesting that opioid-mediated analgesia also influences cognitive aspects of pain. Similarly, Uhelski and Fuchs38 showed that naltrexone reduced avoidance of noxious stimuli, reinforcing the opioid system's role in such behaviors.
The open field test was used to assess locomotor activity and anxiety-like behavior,25 and has been recognized as a tool to capture the affective-motivational dimension of pain.39 Injured animals showed reduced center exploration without changes in total distance traveled, suggesting anxiety-like behavior rather than motor impairment. This aligns with Zhang et al.39 who reported increased thigmotaxis in rodent pain models with preserved locomotion. Although findings in neuropathic models are mixed, several studies report reduced center time after nerve injury.40–42 In our study, neither exercise nor naloxone influenced center exploration, indicating limited effects on this domain.
Preclinical studies show that opioids modulate immune responses by promoting a Th2 cytokine profile, while antagonists disrupt this balance and alter Th1/Th2 dynamics.15,43 Our results expand this evidence by revealing region-specific interactions between opioid signaling and neuroimmune mediators in the central nervous system. Nerve injury increased BDNF levels in the PFC and brainstem, consistent with prior findings.44 Exercise reduced BDNF in both regions, and naloxone prevented this reduction in the PFC, suggesting opioid-dependent modulation. In the brainstem, however, naloxone further suppressed IL-4, IL-10, and BDNF, while only BDNF was reduced by exercise, indicating a disruption of neuroimmune homeostasis rather than a regulatory effect.
These findings point to a dual, region-specific role of the opioid system: sustaining regulatory modulation in the PFC and preserving homeostatic balance in the brainstem.45 Accordingly, naloxone not only blocked exercise-induced analgesia but also interfered with its neuroimmune effects, consistent with previous reports on opioid–cytokine and neurotrophic interactions.15,46,47
We observed no changes in MOR expression in the brainstem or PFC after nerve injury or exercise, contrasting with Kim et al,16 who reported reduced spinal MOR after aerobic training and increased brainstem opioid levels. Differences in regions, protocols, or timing may explain these discrepancies. Despite no correlations between MOR levels and IL-4, IL-10, or BDNF, functional interactions are likely. IL-4 and IL-10 modulate pain by inducing β-endorphin release from immune or glial cells, acting on MORs without necessarily increasing receptor expression.48–51 Likewise, BDNF regulated via CREB and ERK1/2 pathways52 may be modulated downstream of MOR signaling. Morphine-induced BDNF upregulation in microglia, blocked by naloxone, supports this mechanism.53 Overall, our data suggests that low-intensity aerobic exercise engages opioid-dependent pathways to modulate neuroimmune mediators, even in the absence of detectable changes in MOR abundance.
Taken together, these findings suggest that low-intensity aerobic exercise, widely used in clinical practice, can modulate pain through neuroimmune mechanisms, supporting its use as a non-pharmacological strategy for managing chronic neuropathic pain—especially in individuals with low tolerance to high-intensity protocols. Moreover, understanding how the opioid system modulates BDNF and cytokines offers insights into how structured exercise may influence pain perception and neuroplasticity, with potential benefits for functional recovery after peripheral nerve injury.
Among this study’s limitations are the absence of a group treated with a µ-opioid receptor-specific antagonist, the limitations of the model itself in fully replicating human neuropathic pain, and the lack of female subjects, which precludes the analysis of sex differences in neuropathic pain and exercise responses.
ConclusionLow-intensity physical exercise promotes opioid-mediated analgesia and modulates Th2 cytokines and BDNF involved in neuropathic pain. These effects are region-specific and dependent on endogenous opioid signaling, which supports neuroimmune balance in the PFC and prevents dysregulation in the brainstem. Naloxone disrupted these adaptations, highlighting the opioid system’s central role in the beneficial effects of exercise. These findings reinforce exercise as a valuable non-pharmacological tool in rehabilitation strategies for peripheral nerve injury and chronic pain management.
The authors have no conflicts of interest to declare.
The authors thank all collaborators for their contributions and support, which made this work possible. This research received funding from national research and graduate support agencies. Bobinski F received a grant from Anima Institute (CALL No 42/2023). This study was funded by the National Council for Scientific and Technological Development (CNPq, Brazil; Grant No 421556/2018–8). FB and FSS received graduate scholarships from the Support Program for Graduate Education in Community Institutions/Coordination for the Improvement of Higher Education Personnel (PROSUC/CAPES, Finance Code 001). EMFL was supported by a CNPq graduate scholarship. GAT received a CNPq undergraduate research scholarship (PIBIC, CNPq/Brazil). VVH was supported by a postdoctoral fellowship from CAPES (Brazil).