Chronic dizziness has a negative impact on emotional aspects, functional capacity, and quality of life of older people.
ObjectiveTo compare the effects of the conventional Cawthorne & Cooksey and the multimodal Cawthorne & Cooksey protocols on patient-reported outcomes in older adults with vestibular disorders.
MethodsThis is a single-blind, randomized controlled trial with three-months’ follow-up. Older adults with chronic dizziness were randomly assigned to conventional or multimodal protocols. The protocols were performed in individual 50-minute sessions, twice weekly, for two months. The primary outcome was the Dizziness Handicap Inventory (DHI) and the secondary outcomes were the Visual Analogue Scale, the Vestibular Disorders Activities of Daily Living Scale, the Geriatric Depression Scale, and the Activities-specific Balance Confidence Scale. Outcomes were collected at baseline, post-treatment and three-month follow-up; and analyzed on an intention-to-treat approach.
ResultsEighty-two patients were randomized into the conventional (n = 40) or multimodal (n = 42) protocols. There was no between-group difference on DHI at post-treatment (Mean Difference (MD): −0.7; 95% CI: −9.2, 7.8) and at three-month follow-up (MD: −1.6; 95% CI: −9.5, 6.2). No between-group difference was found for the secondary outcomes. All patient-reported outcomes in the within-group analysis showed significant improvement between baseline and post-treatment, and changes were maintained between post-treatment and follow-up. Following treatment, 55% of patients in the conventional and 57% in the multimodal protocol reached DHI clinical improvement (decrease ≥18).
ConclusionsThe addition of multimodal exercises to the conventional Cawthorne & Cooksey protocol did not promote extra benefits on patient-reported outcomes in older adults with chronic dizziness.
Trial registrationAustralian New Zealand Clinical Trials Registry-ANZCTR (ACTRN12610000018011), the trial was registered January 7, 2010 and the first participant was enrolled April 15, 2010. URL of the registry: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=334985.
Vestibular disorders can significantly limit an individual’s ability to function adequately in everyday life.1 Vestibular disorders manifest as a variety of signs and symptoms, including dizziness, vertigo, oscillopsia, and imbalance.2 These symptoms affect individuals physically and psychologically, with negative consequences on activities of daily living (ADL) and quality of life (QoL).2–4
About 40% of older adults cease certain ADL due to dizziness.5 Among 15 ADL, walking close to home was considered the most difficult task to perform by older adults with vestibular disorders.6 Environmental factors (poor lighting, supermarket aisles, and crowded places) can elicit symptoms.1,7 Therefore, around 18.5% of those with vestibular disorders avoid leaving their homes.5 Even during symptom remission, patients are worried about the next episode, preventing movements and performing certain tasks.4 These constraints contribute to the high prevalence of low self-confidence, disability, anxiety, depression, and social isolation in individuals with vestibular disorders.3
Each patient is unique, because the same disease can affect their lives differently.8 Therefore, patient-reported outcomes or self-perceived measures, i.e., health perceptions, QoL, and functional status, are important tools to assess debilitating effects related to vestibular disorders.3 These measures capture patients’ personal experience, which is the main goal for improvement in clinical interventions.8
Vestibular rehabilitation (VR) has been used as a treatment to reduce/eliminate symptoms, improve independence in ADL, and increase self-confidence in patients with vestibular disorders.9 A systematic review with meta-analysis10 supports the use of VR to improve subjective measures of dizziness in people with chronic unilateral peripheral vestibular disorder compared with no treatment. However, no conclusions could be made when different VR protocols were compared.10 Another systematic review of VR determined that while randomized clinical trials with samples of older adults are scarce, positive effects with reduction in vestibular symptoms were reported.11
Despite the positive results of the Cawthorne & Cooksey VR protocol,11 this intervention does not incorporate exercises using different bases of support, strength, or dual tasks, which reflect components required to perform daily activities. In previously published data from this clinical trial, reporting on objective balance control variables, we described that a multimodal VR protocol resulted in superior improvements for two static balance tests (Romberg Sensorial Eyes Closed and Unipedal Stance Left Eyes Open) when compared to the conventional protocol.12 This current paper focuses on reporting patient-related outcomes of QoL, emotional data, and functional capacity. The purpose of this study was to compare the effects of the conventional Cawthorne & Cooksey with the multimodal Cawthorne & Cooksey protocols on patient-reported outcomes in older adults with vestibular disorders after the two-month treatment period and the three-month follow-up. A secondary aim was to investigate clinical factors associated with the primary outcome score.
MethodsThis is a single-blind, randomized controlled trial with a three-month follow-up period (trial registration: ACTRN12610000018011). The study was approved by the Universidade Federal de São Paulo (UNIFESP), Sao Paulo, Sp, Brazil, Research Ethics Committee (no. 1658/09). Details concerning the trial project are described in publications on its study protocol,13 process evaluation14 and effects on balance.12 No deviation from the original design was made after the trial commencement.
Setting and sampleThe sample was recruited from an Otoneurology Outpatient Clinic in which individuals are tested with anamnesis, otolaryngologic examination, and hearing- vestibular tests to determine a diagnosis and etiology of their dizziness. Only older adults (65 years old or over) that received a diagnosis of vestibular disorder from the otoneurologist and confirmed by interview that the symptom was chronic, i.e., dizziness complaint for two months or more after the first occurrence,15 were eligible for this trial. The exclusion criteria were: dizziness not resulting from a vestibular disorder, a diagnosis of benign paroxysmal positional vertigo, cognitive deficits (according to the Mini-Mental State Examination cut-off for education level),16 locomotion with a walker or wheelchair, the practice of regular physical activity (three or more times in the week for more than 30 min in the last two weeks),17 participating in VR treatment in the previous six months, and use of medication for vestibular disorders. Eligible patients provided informed consent to participate in the study.
The sample size was calculated a priori based on the Dizziness Handicap Inventory (DHI) data from a published trial,18 considering an effect size of 14 points and a standard deviation of 20 points. A minimum of 32 patients per group was necessary to ensure a power of 80% and an alpha of 5%. To account for dropout and assure adequate sample size, at least 15% additional patients were recruited to compose the final sample. The final sample consisted of 82 patients who were randomly allocated to the experimental group (multimodal Cawthorne & Cooksey, n = 42) or the control group (conventional Cawthorne & Cooksey, n = 40).
Randomization was performed by blocks using a statistical program. Blocks were gradually performed according to the number of eligible participants on the waiting list and the capacity of attendance of the VR outpatient clinic. As a result, we had different blocks (varying from 4 to 12 individuals) with some of them composed of an odd number of patients and, consequently, unequal groups were formed.
To maintain concealed allocation, the therapist providing the intervention received a telephone call informing about the participant’s allocation group just prior to the first treatment session. Randomization and concealed allocation were performed by a researcher not involved in the recruitment or assessment of the participants.
The sample characteristics are described elsewhere.13 Participants from the two VR groups were similar at baseline for sociodemographic data, health status, and vestibular dysfunction characteristics.13
InterventionThe VR protocols were performed in individual 50-minute sessions, twice weekly, for two months (16 sessions) in a large, quiet, well-lit office (6 m long by 3 m wide). To guarantee that people in both VR protocols received the same amount of stimuli during the session, the exercises for both protocols were monitored by time (approximately 2 min each) rather than number of repetitions. The sessions were delivered by two trained physical therapists (each with eight years of experience) to standardize the VR protocols, each therapist treated participants using either of the two protocols. Participants were excluded from the trial if they had three or more absences during treatment that could not be rescheduled. Both VR protocols have been fully described elsewhere.12
The control group received treatment based on the conventional Cawthorne & Cooksey protocol.19,20 The conventional Cawthorne & Cooksey protocol consists of four stages (lying down - 1 week, sitting - 1 week, standing - 3 weeks, and walking - 3 weeks) in which eye, head, and trunk exercises are performed based on individual symptoms tolerance. The exercises include elements of velocity (slow and quick), movement direction, and visual cues (eyes open or closed) which become more dynamic through the protocol stages. Participants were cued to maintain visual focus during head movement exercises with eyes open.
The experimental group performed the multimodal Cawthorne & Cooksey protocol,12 which adds components of flexibility, cognition, sensory interaction, and muscle strengthening to the same exercises used with the conventional protocol. The modified protocol incorporates changes in the base of support (feet together and tandem) in exercises in standing position. For flexibility, the movement was made in a stretching position; for muscle strength, weight (0.5–1.5 kg) was added to the upper and lower limbs during the exercises. To challenge proprioceptive information, a medium foam was used for participants to stand on, and for cognition the exercises were performed using a dual task protocol.
Participants from both protocols received a booklet with general information about VR, dietary advice, fall prevention, and instructions to perform home exercises daily. After the intervention was completed, participants were advised to continue performing the home exercises for maintenance. All patients from the VR outpatient clinic receive this general information as part of their usual care.
Outcome measuresParticipants went to VR outpatient clinic for a one-hour evaluation to complete the study protocol subjective and objective outcome measures used at baseline, post-treatment, and three-month follow-up with an assessor blinded to group allocation. All patient-reported outcomes were collected by face-to-face interview, interspersed with objective measures to avoid fatigue.
At baseline, we collected data regarding sample characteristics (age, sex, number of comorbidities, number of medications, presence of musculoskeletal pain, topographic vestibular diagnosis, onset of symptoms and type of dizziness). The primary outcome was the DHI total score, which measures self-perceived handicap in patients with vestibular disorders.21 This self-reported questionnaire consists of 25 items, which are answered on a scale and scored as follows: negative response (zero), sometimes (2 points), or affirmative (4 points). The items are divided into physical (7 items / 0–28 points), emotional (9 items / 0–36 points), and functional (9 items / 0–36 points) domains. The total score ranges from zero (no handicap) to 100 points (severe handicap). DHI results were analyzed using the total score, the cut-off score for severe handicap (≥60 points),22 and the minimal clinically important difference (MCID) (a decrease of 18 or more points) reflective of improvement in QoL following treatment.18
Secondary outcome measures included the Visual Analogue Scale (VAS), the Vestibular Disorders Activities of Daily Living Scale (VADL), the Geriatric Depression Scale (GDS), and the Activities-specific Balance Confidence (ABC) Scale.
Participants rated dizziness intensity in the previous 24 h on a 10 cm VAS line, in which zero represented “no dizziness” and 10 represented “severe dizziness”.
The self-perceived impact of dizziness on ADL performance was assessed with the VADL.23,24 The VADL contains 28 activities, which are divided into functional (12 activities), ambulation (9 activities), and instrumental (7 activities) subscales. Each activity is classified by a 10-point qualitative scale as the participant’s self-perception of their performance and independence in the task compared with their execution before developing the vestibular disorder.23 The VADL score is calculated using the median score of the 28 activities, and ranges from one to 10 points, with higher scores indicating greater disability.
The ABC scale was used to evaluate the individual’s self-confidence in their balance while performing daily tasks.25,26 The ABC scale assesses balance confidence in 16 activities in an ascending scale of no confidence (0%) to completely confident (100%), with 10% intervals.26 If the individual does not perform the activity, they must imagine how their balance confidence would be if they had to do the activity. In addition, if the patient needed help to perform the activity (walking aid, object, or person), the answer must consider their confidence using these supports.25 The mean percentage of confidence in all activities was used for data analysis.
The emotional aspect of dizziness was evaluated by the GDS 15-item version.27 The total score ranges from 0 to 15 points, considering one point for each positive response for depressed mood and zero for negative responses. The cut-off score for depression mood (≥5 points) was used in the analyses.27
Data analysisData were analyzed on an intention-to-treat approach with, the last observation carried forward for missing data from withdrawn and dropouts’ participants. Changes in continuous measures were tested by the Repeated Measures Analysis of Variance, in which time (baseline, post-treatment, and three-month follow-up) was the intragroup repeated factor; protocol (conventional and multimodal) was the intergroup factor, and interaction was the relationship of protocols versus time. Changes in the severity level of the DHI (categorical variable) were assessed by the McNemar test for intragroup analysis and the Chi-square test for intergroup analysis. Potential interactions between improvement in treatment based on DHI score (dependent variable, decrease in score of ≥18 points) and sample characteristics (independent variables: age, sex, comorbidities, medications, topographic vestibular diagnosis, pain, onset of symptoms, type of dizziness) were tested by the Mann-Whitney U, Chi-square, or Fisher’s exact tests. The data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) adopting a significance level of 5% for all tests.
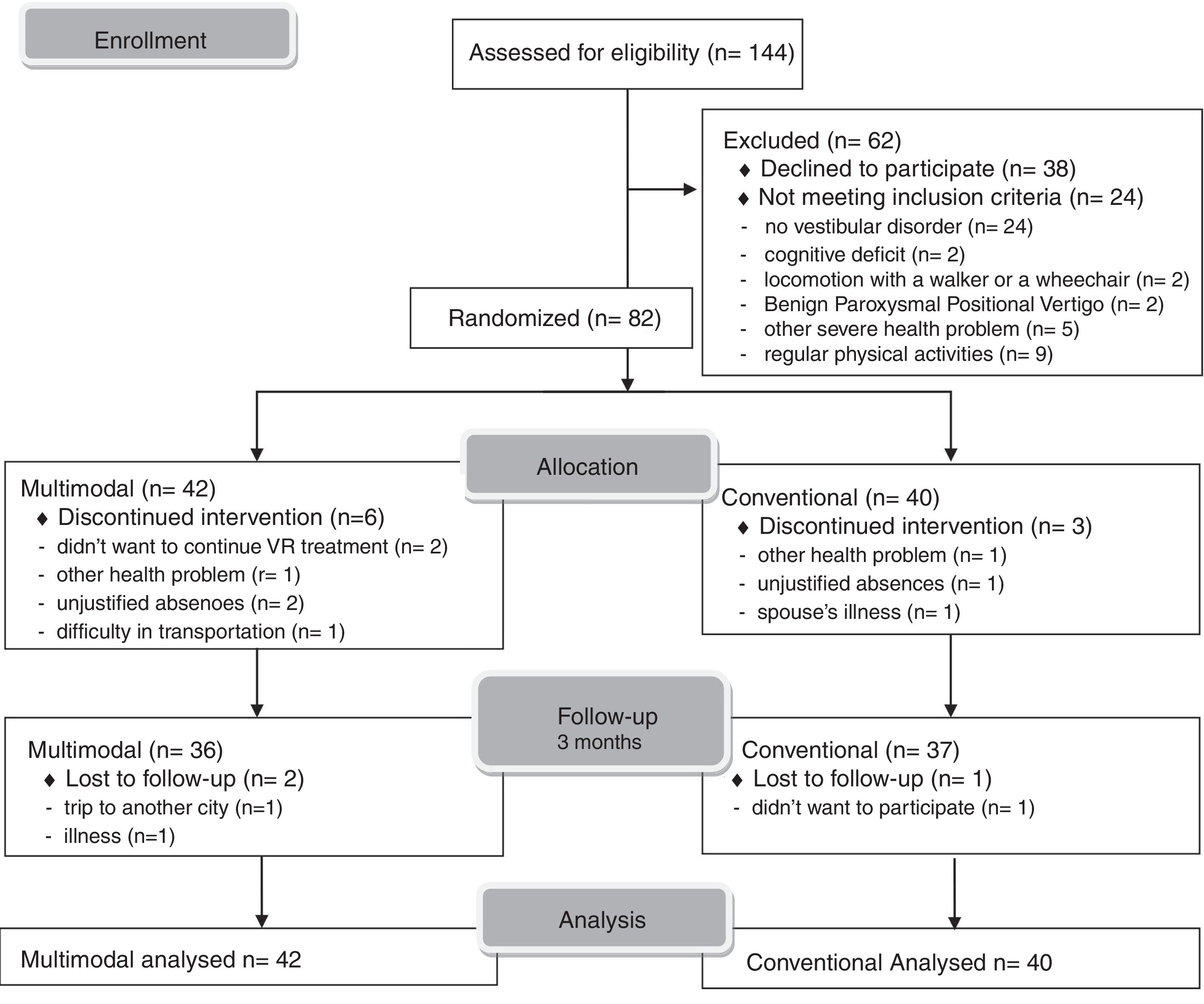
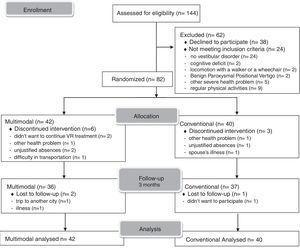
ResultsBetween March 2010 and February 2012, 144 patients with chronic dizziness were referred to VR. Of these patients, 62 were excluded for various reasons, and 82 were eligible for the trial and then randomly assigned to the multimodal (n = 42) or conventional (n = 40) groups. During the clinical trial, 12 patients were withdrawn from the study. Fig. 1 shows the trial flowchart.
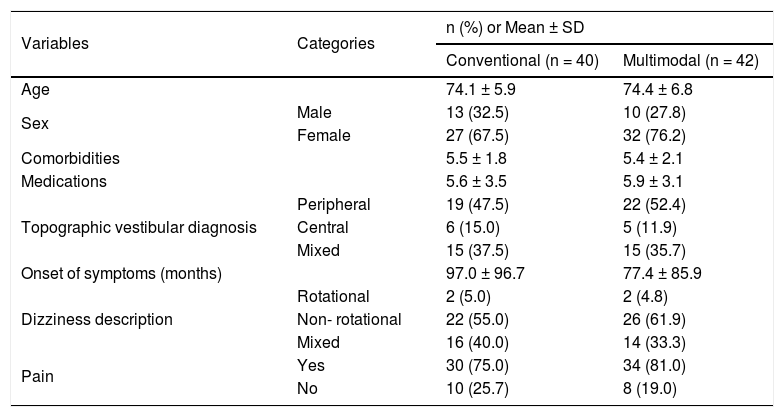
Both groups consisted primarily of women (conventional: 67.5%; multimodal: 76.2%) with advanced age (conventional: 74.1 ± 5.9 years old; multimodal: 74.4 ± 6.8 years) and diagnosis of peripheral topographic vestibular disorder (conventional: 47.5%; multimodal: 52.4%). Sample characteristics, the DHI scores, and scores on secondary outcomes were similar between the VR groups (Table 1 and Table 2).
Characteristics of the participants in the conventional and multimodal protocols at baseline.
| Variables | Categories | n (%) or Mean ± SD | |
|---|---|---|---|
| Conventional (n = 40) | Multimodal (n = 42) | ||
| Age | 74.1 ± 5.9 | 74.4 ± 6.8 | |
| Sex | Male | 13 (32.5) | 10 (27.8) |
| Female | 27 (67.5) | 32 (76.2) | |
| Comorbidities | 5.5 ± 1.8 | 5.4 ± 2.1 | |
| Medications | 5.6 ± 3.5 | 5.9 ± 3.1 | |
| Topographic vestibular diagnosis | Peripheral | 19 (47.5) | 22 (52.4) |
| Central | 6 (15.0) | 5 (11.9) | |
| Mixed | 15 (37.5) | 15 (35.7) | |
| Onset of symptoms (months) | 97.0 ± 96.7 | 77.4 ± 85.9 | |
| Dizziness description | Rotational | 2 (5.0) | 2 (4.8) |
| Non- rotational | 22 (55.0) | 26 (61.9) | |
| Mixed | 16 (40.0) | 14 (33.3) | |
| Pain | Yes | 30 (75.0) | 34 (81.0) |
| No | 10 (25.7) | 8 (19.0) | |
Outcomes of the vestibular rehabilitation protocols at baseline, post-intervention, and three-month follow-up.
| Outcome | Baseline | Post- VR | Follow-up | Baseline – Post- VR | Post- VR – Follow-up | Group | Interaction |
|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean (95% CI) | Mean (95% CI) | Time X Group | ||
| DHI-total score | |||||||
| Conventional | 38.9 ± 18.8 | 20.7 ± 20.0 | 21.5 ± 21.0 | 18.2 (11.9, 24.4) | −0.7 (−6.2, 4.7) | ||
| Multimodal | 47.2 ± 26.4 | 28.3 ± 27.7 | 27.4 ± 27.6 | 18.9 (12.8, 24.9) | 0.9 (−4.9, 6.7) | ||
| Group differences | −0.7 (−9.2, 7.8) | −1.6 (−9.5, 6.2) | |||||
| p-value | <0.01 | <0.01 | 0.96 | 0.12 | 0.85 | ||
| DHI- physical | |||||||
| Conventional | 13.3 ± 6.6 | 6.9 ± 6.8 | 6.5 ± 6.7 | 6.3 (4.2, 8.4) | 0.4 (−1.4, 2.3) | ||
| Multimodal | 14.6 ± 7.6 | 8.5 ± 7.7 | 8.7 ± 8.4 | 6.1 (3.9, 8.2) | −0.1 (−1.8, 1.4) | ||
| Group differences | 0.2 (−2.7, 3.2) | 0.6 (−1.8, 3.1) | |||||
| p-value | <0.01 | <0.01 | 0.83 | 0.23 | 0.80 | ||
| DHI- functional | |||||||
| Conventional | 13.5 ± 8.84 | 8.4 ± 8.0 | 8.3 ± 8.2 | 5.1 (2.6, 7.5) | 0.1 (−1.9, 2.1) | ||
| Multimodal | 19.1 ± 11.3 | 11.3 ± 11.3 | 11.0 ± 11.3 | 7.7 (5.1, 10.3) | 0.3 (−2.2, 3.0) | ||
| Group differences | −2.6 (−6.1, 0.8) | −0.2 (−3.5, 3.0) | |||||
| p-value | <0.01 | <0.01 | 0.77 | 0.05 | 0.20 | ||
| DHI- emotional | |||||||
| Conventional | 12.1 ± 8.1 | 5.4 ± 7.1 | 6.7 ± 8.0 | 6.7 (3.7, 9.7) | −1.3 (−3.4, 0.8) | ||
| Multimodal | 13.5 ± 10.4 | 8.5 ± 10.4 | 7.7 ± 9.7 | 5.0 (2.9, 7.0) | 0.7 (−1.2, 2.7) | ||
| Group differences | 1.7 (−1.7, 5.2) | −2.0 (−4.9, 0.8) | |||||
| p-value | <0.01 | <0.01 | 0.71 | 0.29 | 0.43 | ||
| VAS | |||||||
| Conventional | 3.3 ± 2.3 | 2.0 ± 2.0 | 2.3 ± 2.6 | 1.3 (0.6, 1.9) | −0.3 (−0.9, 0.2) | ||
| Multimodal | 3.4 ± 2.3 | 1.5 ± 1.9 | 1.8 ± 2.3 | 1.90 (1.1, 2.6) | −0.2 (−0.8, 0.2) | ||
| Group differences | −0.58 (−1.5, 0.4) | −0.0 (−0.8, 0.7) | |||||
| p-value | <0.01 | <0.01 | 0.12 | 0.43 | 0.38 | ||
| VADL- total score | |||||||
| Conventional | 4.1 ± 1.7 | 2.8 ± 1.7 | 2.9 ± 1.8 | 1.2 (0.6, 1.8) | −0.1 (−0.5, 0.3) | ||
| Multimodal | 4.2 ± 1.7 | 3.4 ± 1.8 | 3.2 ± 1.9 | 0.8 (0.3, 1.2) | 0.1 (−0.1, 0.5) | ||
| Group differences | 0.4 (−0.3, 1.1) | −0.2 (−0.8, 0.2) | |||||
| p-value | <0.01 | <0.01 | 0.77 | 0.30 | 0.50 | ||
| VADL-functional | |||||||
| Conventional | 3.8 ± 1.9 | 2.8 ± 1.8 | 2.8 ± 2.0 | 0.9 (0.4, 1.5) | −0.0 (−0.5, 0.5) | ||
| Multimodal | 4.2 ± 1.6 | 3.0 ± 1.9 | 3.2 ± 2.0 | 1.2 (0.7, 1.7) | −0.2 (−0.6, 0.2) | ||
| Group differences | −0.2 (−1.0, 0.5) | 0.2 (−0.4, 0.8) | |||||
| p-value | <0.01 | <0.01 | 0.47 | 0.33 | 0.76 | ||
| VADL- instrumental | |||||||
| Conventional | 3.8 ± 2.6 | 2.7 ± 2.1 | 2.8 ± 2.2 | 1.0 (0.2, 1.9) | −0.1 (−0.5, 0.3) | ||
| Multimodal | 4.6 ± 3.0 | 3.2 ± 2.7 | 3.6 ± 2.9 | 1.4 (0.7, 2.0) | −0.4 (−1.0, 0.0) | ||
| Group differences | −0.3 (−1.3, 0.6) | 0.3 (−0.3, 1.0) | |||||
| p-value | <0.01 | <0.01 | 0.09 | 0.17 | 0.65 | ||
| VADL- locomotion | |||||||
| Conventional | 4.5 ± 1.7 | 3.6 ± 1.7 | 3.8 ± 1.9 | 0.9 (0.3, 1.5) | −0.2 (−0.6, 0.1) | ||
| Multimodal | 4.7 ± 1.7 | 3.9 ± 2.1 | 4.0 ± 2.1 | 0.8 (0.3, 1.2) | −0.1 (−0.4, 0.1) | ||
| Group differences | 0.1 (−0.6, 0.8) | −0.0 (−0.5, 0.4) | |||||
| p-value | <0.01 | <0.01 | 0.09 | 0.55 | 0.90 | ||
| GDS | |||||||
| Conventional | 5.9 ± 3.3 | 4.3 ± 3.2 | 4.3 ± 3.6 | 1.6 (0.8, 2.4) | −0.0 (−0.7, 0.6) | ||
| Multimodal | 5.3 ± 3.4 | 3.2 ± 3.0 | 3.6 ± 3.1 | 2.1 (1.3, 2.9) | −0.4 (−0.9, 0.0) | ||
| Group differences | −0.4 (−1.6, 0.6) | 0.4 (−0.4, 1.2) | |||||
| p-value | <0.01 | <0.01 | 0.25 | 0.24 | 0.55 | ||
| ABC scale | |||||||
| Conventional | 59.0 ± 19.6 | 69.9 ± 22.2 | 70.5 ± 23.1 | −10.9 (−15.0, 6.1) | −0.5 (−5.6, 4.5) | ||
| Multimodal | 56.6 ± 23.4 | 73.4 ± 19.9 | 72.6 ± 22.7 | −16.8 (−22.2, −11.3) | 0.8 (−3.0, 4.6) | ||
| Group differences | 5.84 (-1.3, 13.0) | −1.3 (−7.5, 4.8) | |||||
| p-value | <0.01 | <0.01 | 0.93 | 0.80 | 0.24 | ||
ABC, Activities-specific Balance Confidence Scale; CI, Confidence interval; DHI, Dizziness Handicap Inventory; GDS, Geriatric Depression Scale; SD, Standard Deviation; VADL, Vestibular Disorders Activities of Daily Living Scale; VAS, Visual Analogue Scale; VR, Vestibular Rehabilitation.
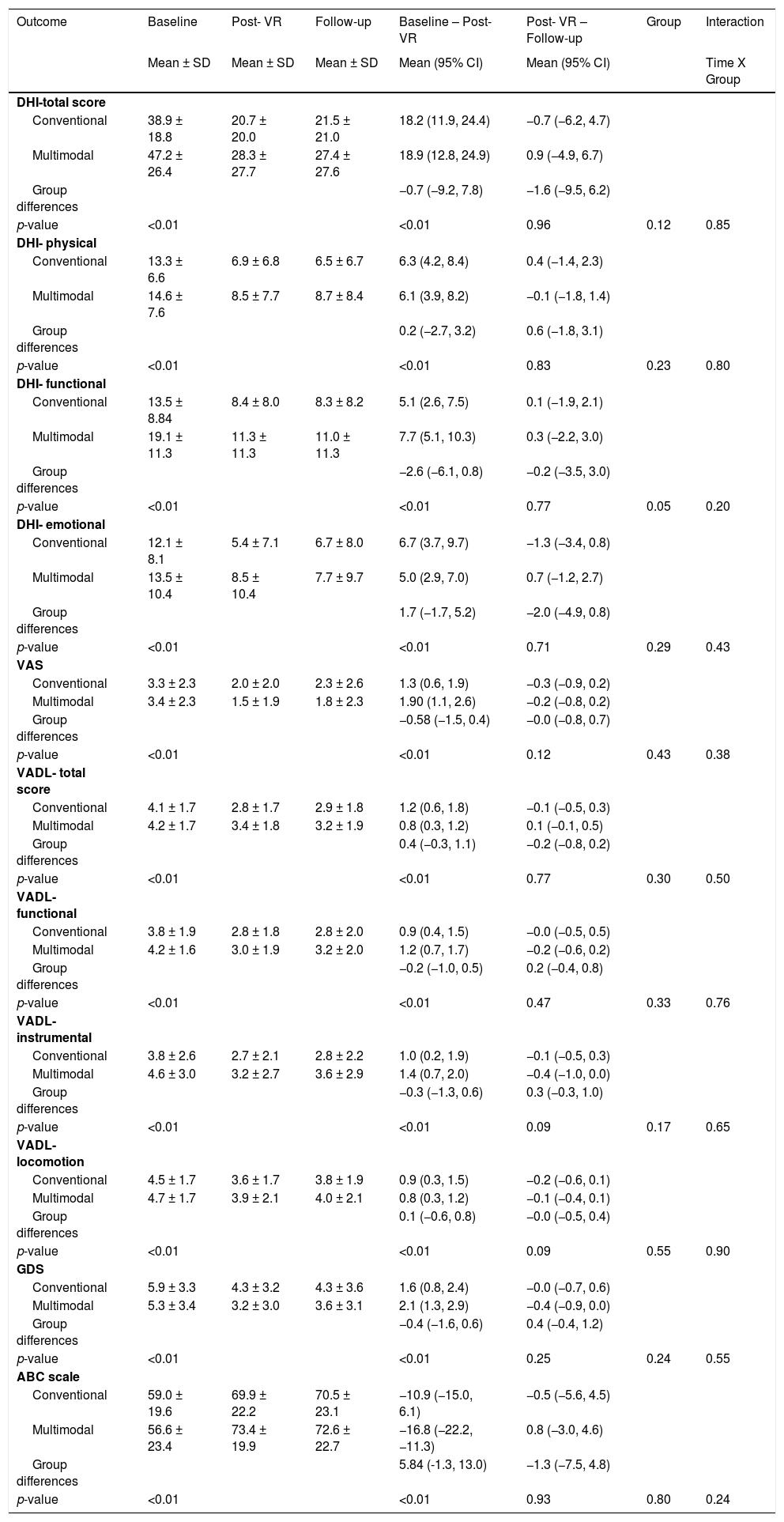
There was no between-group difference on the primary outcome, DHI- total score, after VR (MD: −0.7 95%CI: −9.2, 7.8) and at three-month follow-up (MD: −1.6 95%CI: −9.5, 6.2), i.e. no interaction (differences between group and time points of assessment) (Table 2). In the within-group analysis significant time effects were observed in DHI total score and subscales, i.e., both groups improved after rehabilitation. The post-hoc test showed changes in DHI total score and subscales between baseline and post-treatment and no significant changes between post-treatment and follow-up.
For all secondary outcomes, the between-group analyses showed no differences in changes between VR groups for all time points of assessment (Table 2). The within-group analysis showed significant improvement from baseline to after treatment, which was maintained between post-treatment and follow-up for both VR protocols and for all secondary outcomes (Table 2).
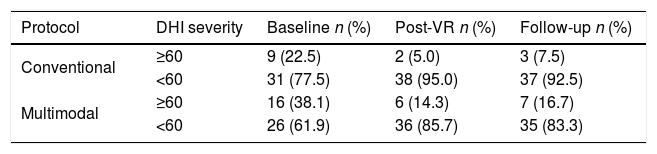
At baseline, nine (22.5%) of the participants in the conventional protocol and 16 (38.1%) of those in the multimodal protocol group presented severe handicap according to a DHI score of ≥60 points. After VR, both groups showed a significant decrease in the number of participants with a severe DHI score (conventional: n = 2, 5.0%, McNemar p = 0.02; multimodal: n = 6, 14.3%, McNemar p = 0.006), which was maintained at follow-up (McNemar p = 1.00) (Table 3). No differences were observed between the VR groups for DHI severity at baseline (Chi-square, p = 0.154), post-treatment (Chi-square, p = 0.265) and follow-up (Chi-square, p = 0.313).
Dizziness Handicap Inventory severity comparison at baseline, post-intervention, and three-month follow-up by Vestibular rehabilitation protocol.
| Protocol | DHI severity | Baseline n (%) | Post-VR n (%) | Follow-up n (%) |
|---|---|---|---|---|
| Conventional | ≥60 | 9 (22.5) | 2 (5.0) | 3 (7.5) |
| <60 | 31 (77.5) | 38 (95.0) | 37 (92.5) | |
| Multimodal | ≥60 | 16 (38.1) | 6 (14.3) | 7 (16.7) |
| <60 | 26 (61.9) | 36 (85.7) | 35 (83.3) |
DHI, Dizziness Handicap Inventory; VR, Vestibular Rehabilitation.
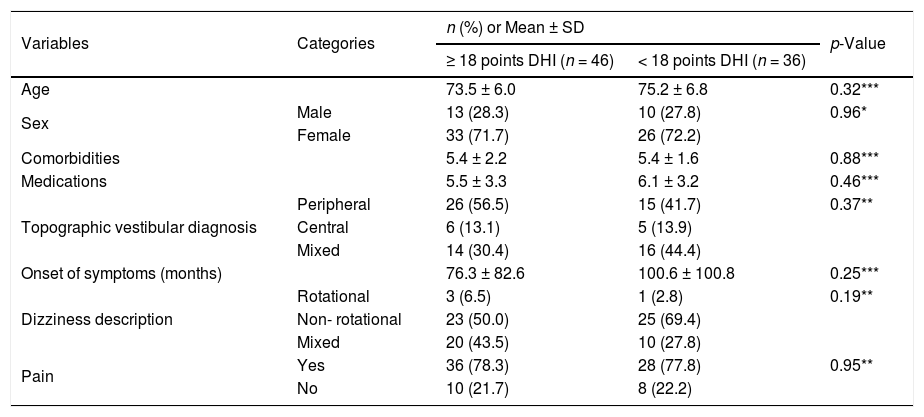
More than half of the patients reached clinical improvement in the DHI (decreased score of ≥18 points) with VR (conventional: n = 22, 55.0%; multimodal: n = 24, 57.1%), and there were no significant differences in changes between protocols (Chi-square, p = 0.845). All participants in the study were used to determine factors that could predict improvement in DHI, with no variables identified (Table 4).
Association between a clinically meaningful improvement score on the Dizziness Handicap Inventory and selected variables after Vestibular rehabilitation in older adults with chronic dizziness (n = 82).
| Variables | Categories | n (%) or Mean ± SD | p-Value | |
|---|---|---|---|---|
| ≥ 18 points DHI (n = 46) | < 18 points DHI (n = 36) | |||
| Age | 73.5 ± 6.0 | 75.2 ± 6.8 | 0.32*** | |
| Sex | Male | 13 (28.3) | 10 (27.8) | 0.96* |
| Female | 33 (71.7) | 26 (72.2) | ||
| Comorbidities | 5.4 ± 2.2 | 5.4 ± 1.6 | 0.88*** | |
| Medications | 5.5 ± 3.3 | 6.1 ± 3.2 | 0.46*** | |
| Topographic vestibular diagnosis | Peripheral | 26 (56.5) | 15 (41.7) | 0.37** |
| Central | 6 (13.1) | 5 (13.9) | ||
| Mixed | 14 (30.4) | 16 (44.4) | ||
| Onset of symptoms (months) | 76.3 ± 82.6 | 100.6 ± 100.8 | 0.25*** | |
| Dizziness description | Rotational | 3 (6.5) | 1 (2.8) | 0.19** |
| Non- rotational | 23 (50.0) | 25 (69.4) | ||
| Mixed | 20 (43.5) | 10 (27.8) | ||
| Pain | Yes | 36 (78.3) | 28 (77.8) | 0.95** |
| No | 10 (21.7) | 8 (22.2) | ||
*Chi-square; **Fisher Test; *** Mann–Whitney U-test.
DHI, Dizziness Handicap Inventory; SD, Standard Deviation.
This randomized clinical trial showed that the addition of multimodal exercises did not lead to greater improvement to patient-reported outcomes than the conventional Cawthorne & Cooksey protocol in older adults with chronic dizziness attributed to vestibular disorders. All five patient-reported outcomes evaluated in this trial improved in both groups following treatment and changes remained after a short period. In this clinical trial, only self-perception measures were chosen, since the cause (dizziness) and consequences (functional incapacity) under investigation are subjective phenomena. It is important to highlight that the self-perceived measures consider the patient’s perspective on their performance,28 and sometimes this perception differ from those of health care professionals and/or family members. Regardless of whether the patient’s responses were over or underestimated compared to actual performance, it is imperative to recognize the way patients feel about the interference of dizziness in their daily lives. Patient performance in a daily activity is not only a matter of being physically able, it is important that older adults feel capable and that the task has meaning when performed.
A systematic review and meta-analysis10 of treatment for chronic unilateral peripheral vestibular hypofunction, which including five clinical trials, determined a standardized mean difference between groups of −0.8 (95% CI: −1.0, −0.6) on the DHI (scale 0 to 4), which means approximately 20 points (scale 0–100) in favor of the experimental group versus the control group without treatment. However, differences were non-significant for the DHI when comparing different VR protocols,10 which is consistent with our results that showed improvement for both groups. One study29 reported that the practice of any type of exercise (light or heavy) among older people with dizziness could reduce the risk of low QoL. Thus, more than the type of VR protocol, encouraging exercise in this population can lead to benefits in QoL and in other problems that come with the dizziness symptom, such as avoiding activities, fear of falling, and depression.
In our trial, the sample characteristics of sex, age, and otoneurologic diagnosis, had no association with DHI clinical improvement. According to the Evidence-Based Clinical Practice Guideline from the American Physical Therapy Association (APTA) older adults have the same potential for improvement with vestibular rehabilitation than adults.30 Another study31 with older adults was also unable to determine any association between QoL using the DHI with the type of dizziness (i.e., rotatory or non-rotatory), post-caloric nystagmus assessment, and topographic vestibular diagnosis. The QoL, in patients with dizziness, is so impaired that it seems to be independent of any specific dizziness characteristics or etiology.32 Because QoL is a multidimensional measure, it is improbable that only one characteristic could affect it as a whole.
All outcomes changed with intervention, and improvement was sustained after three months of follow-up. Any benefit acquired by older adults after an exercise program will last depending on continuous participation in the recommended treatment.33 After supervised exercise therapy, it is important that patients with chronic conditions learn how to self-manage their symptoms.
In this trial, the use of home exercise through a booklet to complement the VR protocols is in accordance with the literature recommendations for the treatment of patients with dizziness.30,34 APTA recommend that patients with chronic peripheral disorders under supervised VR sessions should also perform home exercises, at least 20 min per day.30 A VR booklet presents good cost-effectiveness and can help patients with chronic dizziness cope with their symptoms.34
We altered the conventional Cawthorne & Cooksey protocol to provide more functional task-based exercises for older people, without compromising the benefits of the standard protocol, i.e., low cost, easy to administer, well-tolerated by patients. For vestibular compensation and transfer benefits to everyday life, exercises should incorporate daily situations instead of unattractive laboratory situations.35 Since both protocols presented changes in self-perceived measures, we recommend that the therapist consider the patient’s assessment and preferences when choosing the exercises. Older adults are a heterogeneous population, and complaints of dizziness are unique to each patient, as are the behavioral strategies for recovery, so developing an exclusive VR protocol that works for all patients is highly unlikely.35
Limitations of this research can be that patients and therapists were not blinded to the protocol provided, interviewer presence can bias the patient reports and no genuine control group (placebo or no intervention) was used. It is not feasible to blind those doing or delivering exercises. All tests-questionnaires, even those that are objectives, can be bias with patient learning. Regarding a non-intervention group, our sample of patients had a chronic condition that can worsen over time, so denying them treatment would be unethical.
ConclusionsThis manuscript is the final report of a comprehensive clinical trial12–14 regarding the effects of VR on older people with chronic dizziness due to vestibular disorders. Patients with dizziness often make considerable adjustments to their lives to continue performing ordinary tasks. VR can offer patients a way to overcome negative feelings (depression, anxiety, fear), decrease limitations in their activities, and regain confidence in their balance and sense of self-control.36 Herein, we have shown that, regardless of the protocol used, VR can change patient-reported outcomes (dizziness intensity, dizziness-related symptoms, depression, fear of falling, and independence in daily activities) in older people. However, a more functional task-based exercises, i.e. multimodal exercises, did not result in better outcome than a more conventional VR program. Further studies should compare the number of sessions per week (one session versus two) and supervised versus booklet home-based exercises in this population.
Conflicts of interestThe authors declare no conflicts of interest.
FundingThis study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.