Poor exercise capacity is an important negative prognostic marker in patients with chronic obstructive pulmonary disease (COPD). Heart rate variability (HRV) responses can indicate alterations in cardiac autonomic control. Nevertheless, it remains unclear whether these abnormalities are related to cardiorespiratory responses to exercise in these patients.
ObjectiveTo evaluate whether HRV at rest and submaximal exercise are related to impaired cardiopulmonary responses to exercise in COPD patients.
MethodsFifteen men (66.2±8.7 years) with COPD (FEV1: 55.1±19.2%) were assessed. The R-R interval (RRi) data collection was performed at rest (stand position) and during the six-minute walk test (6MWT). All patients performed a symptom-limited cardiopulmonary exercise test on a cycle ergometer. The HRV changes from rest to submaximal exercise (Δ rest-6MWT) were calculated.
ResultsWe found significant correlations between low frequency (LF) and high frequency (HF) Δ rest-6MWT with Δ oxyhemoglobin saturation by pulse oximetry (r=−0.64 and r=0.65, respectively; p<0.05), minute ventilation/carbon dioxide output relationship from beginning to peak exercise (r=−0.52 and r=0.53, p<0.05), and exercise ventilatory power (r=0.52 and r=−0.53, p<0.05). Interestingly, there was a strong positive correlation (r=0.82, p<0.05) between six-minute walk distance (6MWD) and Δ LF/HF from rest to exercise.
ConclusionHRV analysis in the transition from rest to submaximal exercise is associated with exercise ventilatory and hemodynamic abnormalities in COPD patients. Rehabilitative strategies to improve HRV responses may provide an important tool to clinical practice in these patients.
The reduced exercise capacity of patients with chronic obstructive pulmonary disease (COPD) has been understood as a determinant of health status and an independent prognostic marker. Since it is characterized by airflow limitation, lung hyperinflation, and heterogeneous inflammatory injury to intrathoracic airways,1 abnormalities in pulmonary mechanics have been interpreted as the mechanism that limits exercise tolerance in COPD patients. Moreover, peripheral muscle dysfunction – atrophy, shift in fiber-type, changes in capillarization, poor oxidative capacity, mitochondrial dysfunction, and weakness especially in lower limb muscles – appears as a relevant systemic outcome, whose clinical significance is reflected by exercise tolerance, quality of life, and mortality.2
When it comes to determining COPD pathophysiological alterations and its process to exercise intolerance, cardiopulmonary exercise testing (CPET) is more accurate and sensitive than rest and/or field tests, although it demands higher costs and specialized equipment and staff.3 Derived variables from oxygen consumption (V˙O2), carbon dioxide output (V˙CO2) and minute ventilation (V˙E) (e.g., oxygen uptake efficiency slope – OUES, minute ventilation to carbon dioxide output ratio from beginning of exercise to peak exercise – V˙E/V˙CO2 slope) add relevant physiological information about ventilatory efficiency of numerous chronic diseases.4 Recently, circulatory power (CP, the product of peak V˙O2 and peak systolic blood pressure – SBP) and exercise ventilatory power (EVP; peak SBP/V˙E/V˙CO2 slope ratio) combine ventilatory (in)efficiency with hemodynamics during exercise and are important parameters to determine prognosis in patients with congestive heart failure (CHF).5,6 It remains unclear whether these variables are also clinically relevant to the context of COPD.
It is known that responses to sympathetic and vagal stimuli are depressed in COPD patients.7 Heart rate variability (HRV) analysis consists in an autonomic nervous system evaluation tool with promising clinical approach,8 given that it is a straightforward, readily applicable, and low-cost method. Small reductions in forced expiratory volume in 1s (FEV1) increase cardiovascular risk in COPD patients by two to three times compared to healthy humans.9 In turn, the six-minute walk test (6MWT), a simple and inexpensive test, can be easily carried out to assess high-intensity submaximal exercise capacity in COPD patients.3,10,11 Since most of the daily activities (DA) for these patients are performed at high-intensity submaximal levels, the six-minute walk distance (6MWD) provides data about functional capacity, response to therapy, and prognosis.12
In the literature, the relationship between cardiac autonomic function at rest and variables obtained from CPET in COPD patients is scarce and controversial. Bartels et al.13 assessed cardiac autonomic modulation during ramped bicycle ergometry and showed a significant increase in high-frequency (HF) modulation (reflective of parasympathetic cardiac modulation) with a decrease in the low-frequency (LF)/HF ratio in COPD patients during maximal volitional exercise, which might indicate a loss of the ability to achieve a sympathetic response as baseline sympathetic tone is elevated and/or HF modulation is increased. This is seen in COPD patients but not in healthy control subjects, indicating an abnormal level of parasympathetic tone. On the other hand, even though HRV was related to the level of DA, Camillo et al.14 did not find statistically significant correlations with exercise capacity (both maximal and functional, measured through CPET and 6MWT respectively). The authors speculated exercise intolerance in COPD patients occurs mainly as a result of muscular factors (such as fatigue) and ventilatory factors (such as dynamic hyperinflation) and not cardiovascular factors.14 A recent study15 showed weak correlations between HRV indexes at rest and aerobic capacity in COPD, suggesting that better HRV indexes at rest may be related to better maximal performance. However, the HRV data collection was assessed in the seated position and it is known posture interferes with HRV responses.16 To our knowledge, no data exist regarding correlations between HRV changes from rest to submaximal exercise (both performed in the same position) and variables obtained during a maximal CPET. Thus, the aim of the present study was to investigate whether the use of simple assessment tools, like HRV analysis at rest and submaximal exercise (6MWT), can help to determine impaired cardiopulmonary exercise responses to exercise in COPD patients. Our hypothesis was that the use of non-invasive devices, such as HRV analysis during a simple field test, could be useful to functional evaluation of COPD patients. We believe that heart rate (HR) responses and its variability during field tests may reflect ventilatory and hemodynamic impairment during incremental exercise in these patients.
MethodsSubjectsFifteen sedentary males with smoking-related COPD1 were enrolled in this observational, clinical, cross-sectional study. All patients were evaluated in an outpatient specialized clinic by the same physician and optimally treated before study initiation for at least 3 months (Table 1). All patients presented with a clinical and spirometric diagnosis of COPD (FEV1/forced vital capacity <0.7 and post-bronchodilator FEV1<80% predicted) at stages II, III, or IV.1 All subjects reported suffering from chronic dyspnea (modified Medical Research Council scale score). No decompensation episodes occurred in any enrolled subject for at least one month prior to study initiation and no subjects had participated in a regular physical exercise program during the previous six months. The exclusion criteria included long-term O2 therapy (for at least 6 months), type I or non-controlled type II diabetes mellitus and peripheral vascular disease, orthopedic/rheumatological/neurological conditions that would preclude participation in the protocol, uncontrolled hypertension, other concomitant respiratory diseases, current alcoholism, use of theophylline, history of cardiac arrhythmias or potential electrocardiogram alterations, exercise-induced asthma, unstable angina, acute myocardial infarction during the previous six months, syncope, any contraindication to exercise testing according to American Heart Association guidelines,17 and inability to understand and cooperate with the procedures. Approval was granted by the Medical Ethics Committee of São Paulo Hospital, São Paulo, Brazil (protocol 473.529/2013). All subjects were informed about study objectives, experimental procedures and potential risks and gave written informed consent before study initiation.
Baseline patient characteristics.
| COPD (n=15) | |
|---|---|
| Demographic/anthropometric | |
| Age, yrs | 66.2±8.7 |
| Height, cm | 166.7±0.1 |
| Weight, kg | 68.9±15.5 |
| BMI, kg/m2 | 24.6±4.4 |
| Smoking, ex/current | 15/0 |
| pack-yrs | 48.5±27.8 |
| mMRC, 1/2, n (%) | 10 (66.7)/5 (33.3) |
| Echocardiogram | |
| LVEF, % | 66.8±4.3 |
| RV diameter, mm | 22.0±3.4 |
| LVDD, normal/type I, n (%) | 1 (0.06)/14 (0.94) |
| Comorbidities, n (%) | |
| Hypertension | 8 (53.3) |
| Type 2 diabetes | 2 (20.0) |
| Hypercholesterolemia | 5 (33.3) |
| Chronic kidney disease | 12 (80.0) |
| Alcoholism, ex/current | 4 (26.7)/0 |
| Medications, n (%) | |
| LABA | 1 (6.7) |
| LABA+ICS | 5 (33.3) |
| LABA+ICS+LAMA | 8 (53.3) |
| LABA+LAMA | 1 (6.7) |
| Beta-blockers | 0 |
| ACE inhibitors | 5 (33.3) |
| Amlodipine | 5 (33.3) |
| Spironolactone | 1 (6.7) |
| Amiodarone | 1 (6.7) |
| Furosemide | 1 (6.7) |
| Hydrochlorothiazide | 5 (33.3) |
| Aspirin | 2 (13.3) |
| Statin | 5 (33.3) |
| Metformin | 3 (20.0) |
BMI, body mass index; mMRC, modified Medical Research Council; LVEF, left ventricular ejection fraction; RV, right ventricle; LVDD, left ventricle diastolic dysfunction; LABA, long-acting beta2-agonist; ICS, inhaled corticosteroids; LAMA, long-acting anticholinergics; ACE inhibitors, angiotensin-converting-enzyme inhibitors.
All patients were submitted to a comprehensive evaluation, divided into three days: (1) clinical evaluation by a physician and a physical therapist, followed by lung function tests (resting blood gases, spirometry, static lung volumes – SLV, and diffusion capacity of the lung for carbon monoxide) and Doppler echocardiography; (2) HRV data collection (stand position) and 6MWT; (3) incremental CPET.
MeasurementsAll subjects were evaluated during the same time of the day (from 1pm to 5pm) in order to avoid differences due to circadian rhythm. They were instructed to abstain from caffeinated and alcoholic beverages and to avoid exercise on the day before data collection.
Lung function: Spirometry, gas transfer, and SLV (1085 ELITE DTM, Medical Graphics Corporation, St. Paul, MN, USA) were measured according to American Thoracic Society/European Respiratory Society guidelines.18–20 Resting blood gases were obtained by samples from the radial artery.21
Doppler echocardiography: All individuals were submitted to a 2D echocardiogram using an iE33 system (Philips, Andover, MA, USA) with a 2–5MHz matrix transducer and tissue Doppler imaging software. Patients were studied in the left lateral decubitus position by the same physician. Quantification of the cardiac chambers was performed according to the American Society of Echocardiography.22
Acquisition of R-R interval (RRi): RRi was registered using the Polar® system (Polar® S810i, Kempele, Finland), at rest in the standing position (10min), and during 6MWT. An elastic belt (Polar T31 transmitter, Polar Electro, Kempele, Finland) was attached to the subject's chest at the level of the lower third of the sternum. The belt contains a stable case with HR electrodes, an electronic processing unit, and an electromagnetic field transmitter. The HR signals are continuously transmitted to the Polar Advantage receiver unit via an electromagnetic field.23 All data were transferred to a computer using Polar Pro-Trainer 5TM® software.
HRV analysis: HRV was analyzed using Kubius HRV® software (MATLAB, version 2.1, Kuopio, Finland). The total period of RRi collection was scrutinized and, within a 10-minute period in the standing position and in the final minute of the 6MWT, the most stable noise-independent segment (i.e., without missing data and/or noise events), with at least 256 points,16 was selected for analysis. Time domain, frequency domain, and non-linear analysis were performed at rest and during the 6MWT. The mean RRi, the standard deviation of the normal RR intervals (SDNN), and the square root of the mean squared differences of successive RR intervals (RMSSD) were obtained for time domain linear analysis. LF and HF (normalized units – nu) included frequency domain HRV indices. The LF/HF ratio was calculated to verify the sympathovagal balance.16 Non-linear HRV analysis was performed from SD1 (standard deviation measuring the dispersion of points in the plot perpendicular to the line-of-identity), SD2 (standard deviation measuring the dispersion of points along the line-of-identity), alpha1 and alpha2 (respectively, short and long-term fluctuations of detrended fluctuation analysis), and approximate entropy (ApEn) indices. SD1 is related to parasympathetic activity, while SD2 reflects total variability. Alpha1 and alpha2 were used to quantify the fractal property of the RRi temporal series. In healthy conditions, alpha1 should be close to 1 and higher than alpha2.24 ApEn detects changes in a time series and provides a non-negative number to the series. Higher values indicate higher complexity.25 In order to verify responses due to sympathetic stimuli (exercise), HRV indices were also expressed in delta (Δ) (Δ rest-6MWT=HRV index at rest minus HRV index during 6MWT).
6MWT: Subjects were asked to walk at their own maximal pace, without running, along a 30m perimeter. Patients were allowed to stop, but they could start again, if possible, within the allocated 6min.10 Oxyhemoglobin saturation by pulse oximetry (SpO2 – %, Nonin Medical, Plymouth, MN) and HR were continuously recorded. Breathlessness and leg effort were rated according to the 10-point Borg category-ratio scale at the 6th min.26 The test was performed twice, with at least 30min between tests, and the best 6MWD was recorded.
CPET: Symptom-limited, incremental (5 or 10W) exercise testing was performed on an electronically braked cycle ergometer (Corival 400, Lode BV, Netherlands) using the Vmax229d Cardiopulmonary Exercise Testing System (SensorMedics).27V˙O2, L/min, V˙CO2, L/min, respiratory exchange ratio RER=V˙CO2/V˙O2, V˙E, L/min, tidal volume (VT, ml), end tidal carbon dioxide tension (PETCO2, mmHg), and respiratory frequency (breaths/min) were obtained breath-by-breath and averaged as 20s bins. SpO2 was continuously recorded. Breathlessness and leg effort were rated according to the 10-point Borg category-ratio scale at the peak of exercise.26 The V˙E/V˙CO2 relationship from the beginning of exercise to peak exercise was derived via least squares linear regression (i.e., y=mx+b, m=slope) (Microsoft Excel, Microsoft Corp., Bellevue, WA, USA).28 The OUES was calculated using the log-transformation (base 10) of V˙E (L/min) on the x-axis and V˙O2 (L/min) in the y-axis.29 CP (mmHgmlO2/kg/min) was defined as the product of peak V˙O2 and peak SBP.5 EVP (mmHg) was defined as peak SBP divided by the V˙E/V˙CO2 slope.6
Statistical analysisThe patients’ baseline characteristics and functional variables at rest and at peak exercise are reported as means±standard deviation. According to variable distribution (Shapiro–Wilk test), Pearson's or Spearman's moment correlation coefficients were used to test the association between HRV indices (dependent variables) and 6MWT and CPET variables (independent variables). The magnitude of correlations was determined considering the following classification scheme for r-values: (0.26–0.49: low or weak; 0.50–0.69: moderate; 0.70–0.89: strong or high; 0.90–1.0: very high).30 Considering a moderate association of 0.6, the calculated effect size for the current sample was 0.7.31 Post hoc analysis revealed a power of 0.93 for the current sample at an α level of 0.05 (GPower software, version 3.1.3, Germany). All statistical analysis was conducted at a 95% level of significance. Statistical analysis was performed using the Sigma Plot for Windows, version 11.0 (Sigma Plot, San Jose, CA, USA).
ResultsGeneral characteristicsTwenty-six COPD patients from a specialized outpatient clinic (sample of convenience) were recruited. Eleven did not fulfill the inclusion criteria [long-term O2 therapy (1), recent decompensation episode (4), current alcoholism (1), participation in pulmonary rehabilitation (1), refusal to participate (3), unable to perform movement of cycling (1)]. The patients’ baseline characteristics are shown in Table 1. Table 2 shows functional variables obtained at rest, during 6MWT, and during CPET.
Functional variables at rest and at submaximal and maximal peak exercises.
| COPD (n=15) | |
|---|---|
| Lung function | |
| Forced vital capacity, % pred | 93.1±21.7 |
| FEV1, % pred | 55.1±19.2 |
| FEV1/Forced vital capacity | 44.8±10.7 |
| Total lung capacity, % pred | 112.6±16.4 |
| Inspiratory capacity, % pred | 86.6±24.5 |
| Residual volume, % pred | 163.4±41.4 |
| Raw (cmH2O/L/s) | 3.4±1.1 |
| DLCO, % pred | 55.4±21.1a |
| PaO2, mmHg | 70.5±6.5 |
| PaCO2, mmHg | 37.1±4.9 |
| SaO2, % | 93.2±1.8 |
| 6MWT | |
| Peak HR, bpm | 110±18 |
| Peak SBP, mmHg | 141±24 |
| Peak DBP, mmHg | 78±10 |
| Peak SpO2, % | 86±6 |
| Peak Borg dyspnea score | 2±2 |
| Peak Borg fatigue score | 2±3 |
| 6MWD, m | 465.8±43.9 |
| CPET | |
| Peak workload, W | 75±22 |
| Peak V˙O2, ml/kg/min | 18.7±4.0 |
| Rest PETCO2, mmHg | 32.2±3.7 |
| Peak PETCO2, mmHg | 37.7±7.2 |
| Peak RER | 1.1±0.1 |
| Peak HR, bpm | 136±21 |
| Peak SBP, mmHg | 174±27 |
| Peak DBP, mmHg | 87±12 |
| Peak SpO2, % | 88±5 |
| Peak Borg dyspnea score | 7±3 |
| Peak Borg fatigue score | 7.5±2 |
| V˙E/V˙CO2 slope | 29.1±7.2 |
| OUES | 1.2±0.4 |
| CP, mmHgmlO2kg−1min−1 | 3151.9±1006.0 |
| EVP, mmHg | 6.5±2.7 |
FEV1, forced expiratory volume in 1s; Raw, airway resistance; DLCO, carbon monoxide diffusing capacity; PaO2, arterial partial pressure for oxygen; PaCO2, arterial partial pressure for carbon dioxide; PETCO2, end-tidal partial pressure for CO2; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; 6MWD, six-minute walk distance; RER, respiratory exchange ratio; OUES, oxygen uptake efficiency slope; CP, circulatory power; EVP, exercise ventilatory power.
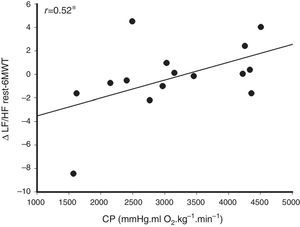
Several moderate, statistically significant correlations ranging from −0.53 to −0.65 (not shown) were observed between CPET variables (peak relative V˙O2, peak SpO2, peak SBP, peak DBP, and peak VT) and ΔHRVrest-6MWT (ΔHF, ΔLF, ΔSD2, and ΔLF/HF). Other positive, moderate, statistically significant correlations ranging from 0.58 to 0.65 (not shown) were also found between CPET variables (peak relative V˙O2, peak SpO2, and peak DBP) and ΔHRVrest-6MWT (ΔHF, ΔLF, Δα1, and ΔLF/HF). Linear indices (LF and HF) correlated with CPET variables obtained directly (ΔSpO2, respectively, r=−0.64 and r=0.65, p<0.05) and indirectly (V˙E/V˙CO2 slope, respectively, r=−0.52 and r=0.53, p<0.05; EVP, respectively, r=0.52 and r=−0.53, p<0.05). Moderate correlations were observed between alpha1 (Δrest-6MWT) and ΔSpO2, OUES, and EVP (respectively, r=−0.56, r=0.51 and r=0.57, p<0.05). Fig. 1 illustrates the positive, moderate correlation between the CP and ΔLF/HF rest-6MWT (r=0.52, p<0.05).
Relationship between sympathovagal balance variation (Δ low- to high-frequency ratio – LF/HF) from rest to submaximal exercise (during the six-minute walk test – 6MWT) with hemodynamic responses during maximal exercise testing represented by circulatory power (product of peak oxygen uptake and peak systolic blood pressure). This result suggests that the modulation of heart rate responses on submaximal intensities is linked to cardiovascular responses during maximal exercise testing. *p<0.05.
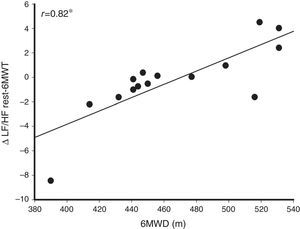
Table 3 shows the correlation coefficients between 6MWT variables and HRV indices obtained at rest, during exercise (6MWT), and in the transition from rest to submaximal exercise (Δrest-6MWT). A strong, statistically significant correlation (r=0.82, p<0.05) was observed between 6MWD and ΔLF/HF rest-6MWT (Fig. 2).
Correlation coefficients between HRV indices (at rest, during exercise, and in the transition from rest to submaximal exercise) and 6MWT variables.
| 6MWT variable | Coefficient | |
|---|---|---|
| Rest | ||
| LF | Peak DAP | 0.80* |
| LF (n.u.) | ΔDAP | 0.71* |
| HF (n.u.) | ΔDAP | −0.71* |
| LF/HF | Peak Borg dyspnea score | 0.54* |
| SD2 | Peak DAP | 0.75* |
| Alpha1 | Peak Borg dyspnea score | 0.53* |
| Peak Borg fatigue score | 0.53* | |
| ΔDAP | 0.64* | |
| ApEn | 6MWD | −0.64* |
| Peak DAP | −0.63* | |
| Submaximal exercise | ||
| RRi | Peak O2 saturation | 0.54* |
| LF (n.u.) | 6MWD | −0.53* |
| HF (n.u.) | 6MWD | 0.55* |
| Alpha2 | Peak HR | 0.58* |
| ApEn | 6MWD | −0.52* |
| ΔRest-6MWT | ||
| LF (n.u.) | 6MWD | 0.58* |
| HF (n.u.) | 6MWD | −0.59* |
| Alpha1 | 6MWD | 0.62* |
HRV, heart rate variability; 6MWT, six minute walk test; LF, low frequency; HF, high frequency; n.u., normalized units; LF/HF, low-frequency/high-frequency ratio; SD2, standard deviation measuring the dispersion of points along the line-of-identity; alpha1, short term fluctuations of detrended fluctuation analysis; ApEn, approximate entropy; RRi, R-R interval; alpha2, long-term fluctuations of detrended fluctuation analysis; DAP, diastolic arterial pressure, mmHg; ΔDAP, peak DAP minus rest DAP; 6MWD, six-minute walk distance; HR, heart rate, beats per minute; Δ rest-6MWT, HRV index at rest minus HRV index during the 6MWT.
Strong relationship between sympathovagal balance variation (Δ low to high frequency ratio – LF/HF) from rest to submaximal exercise (during the six-minute walk test – 6MWT) with walking distance covered. This data suggests that the modulation of heart rate response is linked to the performance of COPD patients. *p<0.05.
The main findings of this study showed that, in moderate-to-severe COPD patients, (1) CPET variables correlated moderately with linear and nonlinear HRV indexes (Δrest-6MWT), (2) the HRV indices (linear and nonlinear) at rest and during the 6MWT correlated moderately/strongly with variables obtained during the 6MWT, and (3) a strong relationship between 6MWD and ΔLF/HF rest-6MWT.
Association between CPET variables and HRV indicesLeite et al.15 demonstrated resting HRV assessment can become a tool to predict exercise capacity in COPD patients. The authors showed that autonomic cardiac function assessment at rest (sitting position) had a weak, although statistically significant, correlation with variables obtained during CPET performed on a treadmill. Since posture interferes with HRV,16 the patients in the present study were assessed at rest, in the same posture in which 6MWT was carried out, i.e., standing position. In addition, we believe that HRV indices expressed in delta (Δrest-6MWT) characterize HRV response to a given stimulus (sympathetic), which is coherent with the expected response during CPET. Therefore, many correlations were found between CPET variables and HRV indices Δrest-6MWT.
The positive SpO2 variations (i.e., Δ>0) in this sample correlated with more adequate cardiac autonomic responses (ΔLF<0 and ΔHF>0) due to sympathetic stimulation (exercise). Since exercise-related oxygen desaturation is common in COPD patients as the disease advances4 and given that hypoxia has been implicated as a direct cause of peripheral nerve damage in COPD,32 this might explain why those who presented desaturation during exercise also showed poorer cardiac autonomic responses than those who did not. Statistically significant correlations between EVP and ΔLF and ΔHF showed adequate response of both frequency bands, from rest to submaximal exercise, corresponded to lower EVP values, and therefore poorer response to aerobic capacity. It seems to be a contradictory relationship; however, most of the subjects included in this study (n=13) presented with signs of air trapping at rest (augmented residual volume – RV – and RV/total lung capacity ratio) and we speculate they also presented with dynamic hyperinflation during exercise. Therefore, we might infer the elevated starting volume and also high work of breathing at rest and during exercise could have caused parasympathetic overactivation and reset the autonomic cardiac control to a new level, causing a “false positive” response from rest to exercise. In addition, it is known that the V˙E/V˙CO2 slope reflects disease severity and its prognosis, with values below 30 being considered normal.4 EVP combines ventilatory efficiency with systemic hemodynamics during exercise (peak SBP/V˙E/V˙CO2 slope ratio), two important physiologic measures related to the ability to respond to aerobic exertion. Impaired EVP reflects deteriorating right heart function and pulmonary hemodynamics33 and, in CHF patients, an EVP below 3.5mmHg is a strong independent predictor of cardiac events.6 Although an EVP threshold has not been established for COPD patients, the majority of patients in our sample presented with low EVP values, possibly due to a high V˙E/V˙CO2 slope (above 30) suggesting poor response to aerobic exercise.
The current results showed alpha1 changes from rest to submaximal exercise correlated with ΔSpO2, OUES, and EVP. The causes of these relationships cannot be elucidated in this study, thus deserve further investigation; however, in the elderly population, changes in alpha1 characterize a powerful predictor of sudden cardiac death, whose causes appear to be explained by sympathetic overactivation.34 OUES, an objective, effort-independent measure to estimate cardiopulmonary functional reserve, may be influenced by changes in partial pressure of CO2, V˙CO2, and physiological dead space,29 abnormalities present in COPD patients.3 It seems the analysis of nonlinear HR dynamics from rest to submaximal exercise may provide relevant clinical information regarding greater hypoxemia and both lower ventilatory efficiency and lower ventilatory power during exercise in moderate-to-severe COPD patients.
In patients with heart disease, functional status can be assessed using CP, a parameter that summarizes the responses of HR, left ventricular systolic volume, arterial blood pressure, and arteriovenous oxygen difference to exercise.5 In CHF patients, the peak CP seems to be a better prognostic marker than peak V˙O2 alone.5 In the present study, patients with higher CP values presented positive ΔLF/HF rest-6MWT (Fig. 1), which may represent sympathovagal balance changes from rest to exercise, situation in which sympathetic predominance and reduced vagal stimulation are expected responses.
Association between 6MWT variables and HRV indicesA widespread test because of its simplicity, convenience, ease of performance, and low cost, the 6MWT provides important clinical information as the severity of COPD increases.3 The study of Pinto-Plata et al.35 showed 6MWD as a better mortality predictor than other COPD severity markers, such as FEV1 and body mass index. Reduced 6MWD represents a fundamental daily inactivity marker in COPD.36 Our study revealed that HRV measurement at rest can help to infer exercise capacity in COPD patients. Both linear (LF/HF) and nonlinear (SD2) HRV indices correlated with subjective data obtained during 6MWT (peak breathlessness) (Table 3). Although van Gestel et al.37 did not observe a correlation between resting HRV and the variables obtained during the 6MWT, the authors suggested that resting HR can be an independent predictor of exercise capacity in COPD patients.
Possibly the most clinically relevant data of our study involved the HRV complexity analysis using the ApEn index. The loss of HRV complexity characterizes greater regularity in RRi dynamics, which reflects poorer adaptability to physiological stress.38 Our results showed moderate statistically significant negative correlation between ApEn and 6MWD, both at rest and during exercise (Table 3), which may indicate that a system with loss of complexity, i.e., with greater regularity and predictability, interferes with exercise performance. We also suggest checking changes in linear HRV indices (LF and HF) Δrest-6MWT (Table 3) for additional information to infer exercise capacity in COPD patients. The strong correlation observed between sympathovagal balance (ΔLF/HF rest-6MWT) and 6MWD (Fig. 2) suggests the faulty adjustment in cardiac autonomic control might be one of the contributors to poor exercise performance in COPD patients.
Clinical implicationsOur results add clinically relevant information to exercise capacity appraisal and cardiac autonomic function assessment in COPD patients. We believe that not only HRV analysis at rest but also its investigation after stimulus (e.g., exercise sympathetic stimulation from 6MWT) may reflect exercise ventilatory and hemodynamic abnormalities as well as contribute to relevant information concerning cardiac autonomic nervous system modulation. We suggest that HRV complexity analysis using the ApEn index can compose HRV evaluation at rest, in which a less complex system (i.e., smaller values of ApEn) correlates with poor 6MWT performance. It is worth highlighting this trend can be modified after six weeks of aerobic exercise training in COPD patients.39
LimitationsNaturally, the present study has limitations. We were able to include a small number of patients; however, the post hoc analysis reveals a power of 0.93 for the present sample. In addition, they were all men and we would need a larger sample size to evaluate the impact of gender differences on HRV and exercise capacity in COPD. For this reason, the current results cannot be applied to female COPD patients, which might limit the external validity of the present study, although it also limits the potential variability between men and women with COPD. In addition, we acknowledge that including an analysis with GOLD Stage 1 COPD patients would have been valuable and could influence the initial management of the disease. We also recognize that the addition of a control group might have added comparative information to our results. Finally, the causes of the alpha1 relationship with SpO2, OUES, and EVP could not be fully clarified by the results presented here, therefore conclusions about this nonlinear index are hypothetical at this time.
ConclusionIn summary, our results showed that the use of non-invasive measures, such as HRV analysis during simple field tests, can be useful in the functional assessment of COPD patients, since cardiac autonomic nervous system modulation to submaximal exercise may reflect exercise ventilatory and hemodynamic abnormalities and therefore infer exercise capacity. Future studies are needed to elucidate physiological mechanisms that explain cardiac autonomic function at rest and exercise performance linkage in a larger group of moderate-to-severe, male and female COPD patients.
Conflict of interestThe authors declare no conflicts of interest.