Warm water immersion therapy (WWIT) has been widely used in the treatment of various clinical conditions, with analgesic and anti-inflammatory effects. However, its mechanism of action has not been fully investigated. The present study analyzed the role of spinal inhibitory neuroreceptors in the antihyperalgesic effect of WWIT in an experimental model of inflammatory pain.
MethodsMice were injected with complete Freund’s adjuvant (CFA; intraplantar [i.pl.]). Paw withdrawal frequency to mechanical stimuli (von Frey test) was used to determine: (1) the effect of intrathecal (i.t.) preadministration of naloxone (a non-selective opioid receptor antagonist; 5 µg/5 µl), (2); AM281 (a selective cannabinoid receptor type 1 [CB1] antagonist; 2 µg/5 µl), (3); and 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; a selective adenosine A1 receptor antagonist; 10 nmol/5 µl), on the antihyperalgesic (pain-relieving) effect of WWIT against CFA-induced hyperalgesia.
ResultsIntrathecal naloxone, AM281, and DPCPX significantly prevented the antihyperalgesic effect of WWIT. This study suggests the involvement of spinal (central) receptors in the antihyperalgesic effect of WWIT in a model of inflammatory pain.
ConclusionsTaken together, these results suggest that opioid, CB1, and A1 spinal receptors might contribute to the pain-relieving effect of WWIT.
Inflammatory and neuropathic pain represent major pain categories and involve increased afferent signaling, increased pain transmission, and altered central pain integration and modulation. Significant advances in understanding pain signaling mechanisms and the neurophysiology of pain have occurred in the past decades. This process included investigation of the diversity of agents and mechanisms that modulate pain signal in both peripheral and central components.1
Under normal physiological conditions, nociceptive signals are produced by intense chemicals, heat, and pressure stimulation of primary afferent sensory A and C nerve fiber terminals.2 These signals are transmitted to superficial layers of the dorsal spinal cord where they undergo substantial modulation by local mechanisms, as well as by projections from supraspinal structures, which can provide both inhibitory and facilitatory influences. From there, these now “modulated” nociceptive signals are further transmitted to brainstem and thalamic sites, and subsequently to the cerebral cortex.3
The dorsal spinal cord expresses a number of receptors for neurotransmitters that can inhibit pain transmission. These receptors are synthesized in the cell body of dorsal root ganglia cells and transported centrally to reside presynaptically on primary afferent neurons.4 Among the inhibitory neuroreceptors expressed in the spinal cord, G protein-coupled receptors such as opioid, cannabinoid, and adenosinergic receptors have recently been extensively studied.2
The central effects of opioids on pain transmission by actions within the dorsal horn of the spinal cord, brainstem, and other supraspinal sites have long been recognized. Spinal activity has also been implicated in opioid analgesia, by the demonstration that intrathecal (i.t.) delivery of opioids produced prominent analgesia.5 Cannabinoid receptor type 1 (CB1) receptors are widely distributed, being present on peripheral and central terminals of sensory afferent neurons, on second-order spinal dorsal horn neurons, in the brainstem, and in brain regions involved in sensory and emotional responses to pain.6 Adenosine A1 receptor (A1R) stimulation is sufficient to diminish pain hypersensitivity in human and animal models of pathological pain.7 Furthermore, there is a body of literature that implicates spinal A1R in antinociception in various pain models.8 Thus, spinal inhibitory neuroreceptors operate as a ‘gate’ for the flow of nociceptive sensory information from the periphery through the spinal dorsal horn to higher brain areas.9
Warm water immersion therapy (WWIT) is a long-used approach to the treatment of chronic disorders and its benefits have been confirmed through systematic review.10 The clinical use of WWIT, as part of physical therapy for inflammatory pain treatment, requires detailed knowledge of the endogenous mechanisms behind WWIT-induced analgesia. Our research groups have recently demonstrated that the antiallodynic effect of WWIT in a model of persistent inflammatory pain is mediated, at least in part, through peripheral opioid, adenosine (A1), and cannabinoid (CB2) receptors.11 In the present study, we demonstrate the role of spinal opioid, cannabinoid (CB1), and adenosine (A1) receptors in the pain-relieving effect of WWIT in a mouse model of inflammatory pain.
MethodsAnimalsExperiments were conducted using male Swiss mice (25−35 g, body weight), housed at 22 ± 2 °C, in a 12-h light/dark cycle (lights on at 6:00 AM), with access to food and water ad libitum. Animals were acclimatized to the laboratory for at least 1 h before the experiments and were used only once. The experiments were performed after protocol approval by the Institutional Ethics Committee (13.006.4.08.IV) of Universidade do Sul de Catarina, Palhoça, Santa Catarina, Brazil and were conducted in accordance with current guidelines for the care of laboratory animals and the ethical guidelines for investigations of experimental pain in conscious animals.12
DrugsThe following substances were used: dimethyl sulfoxide (DMSO), ethanol, complete Freund’s adjuvant (CFA) (Sigma™, St. Louis, MO, USA); 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), and naloxone hydrochloride (Tocris Bioscience™, Ellisville, MO, USA); and AM281 (Cayman Chemical™, Ann Arbor, MI, USA). DPCPX was dissolved in saline with DMSO at 5% to eliminate any effect of the injection. AM281 was dissolved, immediately before use, in DMSO and ethanol, in an amount that did not exceed final concentrations of 1% and 2.5%, respectively.
Warm water immersion therapyAnimals were placed in a plastic box divided into eight compartments (170 × 100 mm), filled with 5.5 l of water to produce a shallow-water environment (3 cm depth). Acclimatization consisted of exposing the animals of all study groups to the environment (shallow water at ∼25 °C) for 3 min once a day on the first, second, and third days before CFA injection.11 During the actual trials, the animals in the control and WWIT groups were exposed to shallow water at 25 °C or 35 °C for 10 min, respectively. The temperature was monitored every 3 min11 with a Thermal Image Camera Testo 880™ (Lenzkirch, Germany) and was kept constant as the test boxes were isolated with an external 25 mm styrofoam layer. After the experiments, the animals were gently dried with a cloth towel.11
CFA-induced inflammation and mechanical hyperalgesiaThe right hindpaw of the mice in the control and WWIT groups were injected (intraplantar [i.pl.]) with 20 µl of an 80% CFA (Mycobacterium tuberculosis) solution (diluted in phosphate-buffered saline) as previously described.13 To assess the effects of WWIT, the animals were subjected to 10 min of shallow “immersion”, 24 h after CFA i.pl. injection. Water level was at a 3 cm depth reaching the animals’ ankle; and was kept at 25 °C for the control group, and 35 °C for the WWIT intervention group. Mechanical hyperalgesia was evaluated 30 min after treatments.11 Mechanical hyperalgesia was assessed as the percentage of paw withdrawals in response to a series of 10 consecutive stimulus with a .6 g von Frey monofilament (VFH, Stoelting™, Chicago, IL, USA) applied through the cage’s mesh floor to the plantar aspect of the animals’ right hindpaw.
Intrathecal injectionsIntrathecal injections were given to fully conscious mice using the method previously described.14 Briefly, the animals were manually restrained, the dorsal fur was shaved, the spinal column was arched, and a 30-gauge needle was inserted into the subarachnoid space between L4 and L5 vertebrae. Correct intrathecal positioning of the needle tip was confirmed by a characteristic tail-flick response. Test agent (5 µl) was slowly injected with a 25 µl Hamilton microsyringe (Hamilton, Birmingham, United Kingdom). In control group animals, a constant volume of 5 µl of saline or vehicle was injected simultaneously. Intrathecal injections were given over a period of 5 s.14
Role of spinal inhibitory neuroreceptors on the antihyperalgesic effect of WWITTo evaluate some of the spinal mechanisms involved in WWIT-induced antihyperalgesia, the animals were treated with classic system antagonists. Drug doses were based on previous studies.11,13,15–17 To assess the participation of opioid spinal receptors in the antihyperalgesic effect of WWIT, the animals received an i.t. injection of naloxone (a nonselective opioid receptor antagonist; 5 µg/5 µl/site) or saline solution (5 µl/site).15 After 15 min, the animals were subjected to Control shallow water immersion or WWIT for 10 min. Mechanical hyperalgesia was evaluated with the von Frey filament test 30 min after water immersion. For this experiment, mice subjected to i.pl. CFA injection were subdivided into the following groups (n = 8 per group): (1) Saline (5 µl/i.t.) + Control shallow water immersion, (2) Naloxone (5 µg/5 µl/i.t.) + Control shallow water immersion, (3) Saline (5 µl/i.t.) + WWIT, and (4) Naloxone (5 µg/5 µl/i.t.) + WWIT.
To assess the possible contribution of cannabinoid (CB1) spinal receptors activation in the antihyperalgesic effect of WWIT, mice received an i.t. injection of AM281 (a selective cannabinoid receptor antagonist; 2 µg/5 µl/site) or vehicle solution (5 µl/site).16 After 15 min, the animals were subjected to Control shallow water immersion or WWIT for 10 min. Mechanical hyperalgesia was evaluated with the von Frey filament 30 min after water immersion. For this experiment, mice subjected to i.pl. CFA injection were subdivided into the following groups (n = 8 per group): (1) Vehicle (5 µl/i.t.) + Control shallow water immersion, (2) AM281 (2 µg/5 µl/i.t.) + Control shallow water immersion, (3) Vehicle (5 µl/i.t.) + WWIT, and (4) AM281 (2 µg/5 µl/i.t.) + WWIT.
We also investigated the involvement of adenosine (A1) spinal receptors in the antihyperalgesic effect of WWIT. The animals received an i.t. injection of DPCPX (a selective A1 receptor antagonist; 10 nmol/5 µl/site) or vehicle solution (5 µl/site).17 After 15 min, the animals were subjected to Control shallow water immersion or WWIT for 10 min. Mechanical hyperalgesia was evaluated with the von Frey filament test 30 min after water immersion. For this experiment, mice subjected to i.pl. CFA injection were subdivided into the following groups (n = 8 per group): (1) Vehicle (5 µl/i.t.) + Control shallow water immersion, (2) DPCPX (10 nmol/5 µl/i.t.) + Control shallow water immersion, (3) Vehicle (5 µl/i.t.) + WWIT, and (4) DPCPX (10 nmol/5 µl/i.t.) + WWIT.
Statistical analysisThe Shapiro–Wilk normality test was applied to evaluate the normality of the data. Descriptive data are expressed as means ± standard deviation (SD) for continuous variables. For each condition (Figs. 1, 2 or 3) the comparisons between experimental and control groups were performed by two-way ANOVA followed by Tukey HSD test for post hoc comparison. P-values (P < .05) were considered as indicative of significance. Statistics were calculated using the Graph-Pad Prism 6.0 software(™) (San Diego, CA, USA).
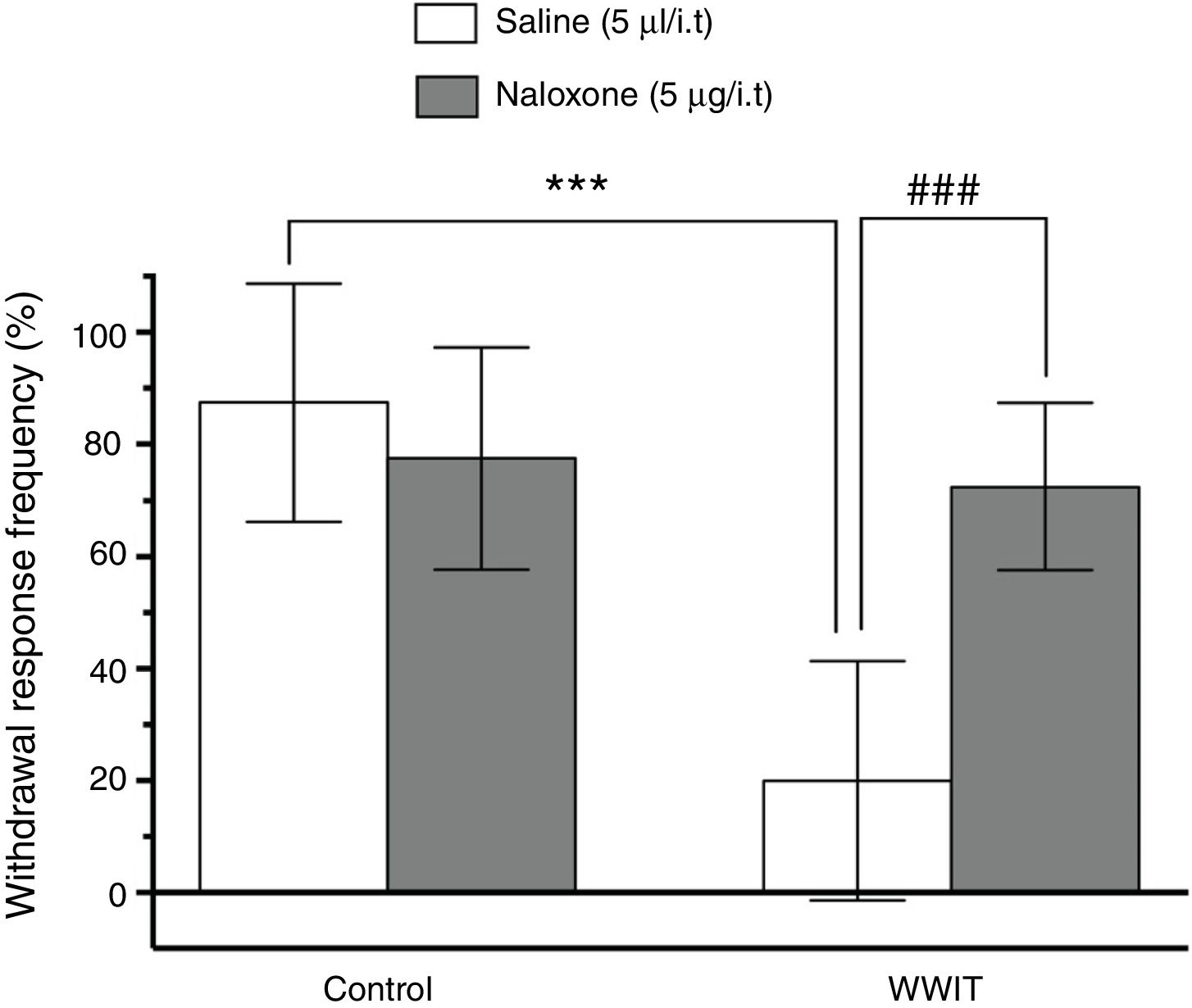
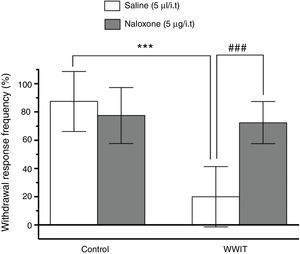
Effect of opioid spinal receptors in the antihyperalgesic effect of WWIT. Mice’s CFA injected hindpaw after treatment in control water 25 °C immersion + Saline i.t. (white bar) or WWIT 35 °C (white bar). Half the mice received an i.t. injection of naloxone prior to control water 25 °C immersion (gray bar) or WWIT 35 °C (gray bar). Results show the antihyperalgesic effect of WWIT 35 °C and how injection of naloxone nullify that effect. Each point represents the mean of eight animals; vertical lines show SD. WWIT, warm water immersion therapy; ***P < .001 and ###P < .001.
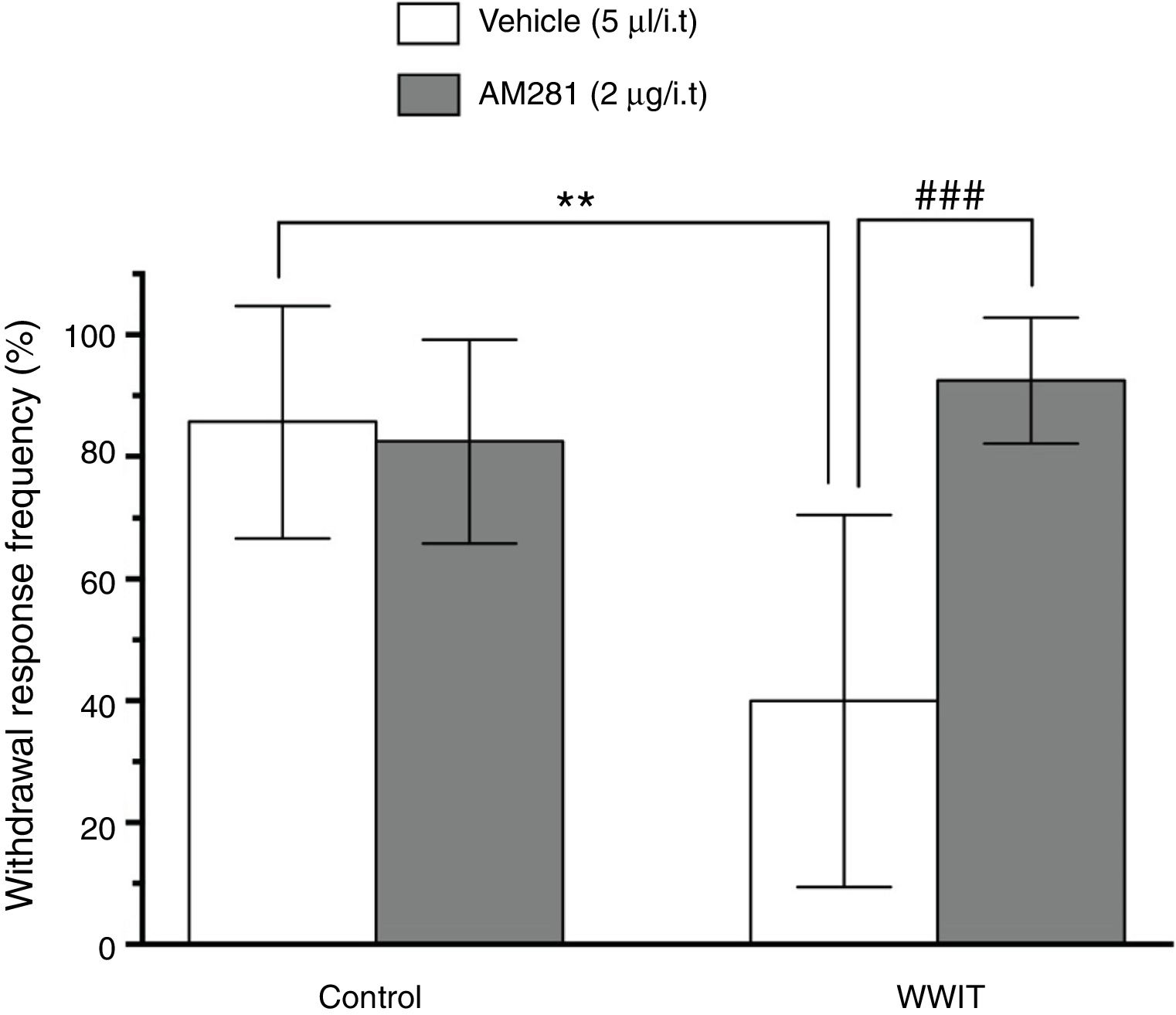
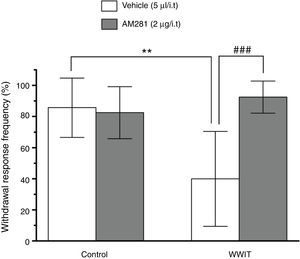
Effect of cannabinoid spinal receptors in the antihyperalgesic effect of WWIT. Mice’s CFA injected hindpaw after treatment in control water 25 °C immersion + Saline i.t. (white bar) or WWIT 35 °C (white bar). Half the mice received an i.t. injection of AM281 prior to control water 25 °C immersion (gray bar) or WWIT 35 °C (gray bar). Results show the antihyperalgesic effect of WWIT 35 °C and how injection of naloxone nullify that effect. Each point represents the mean of eight animals; vertical lines show SD. WWIT, warm water immersion therapy; **P < .01 and ###P < .001.
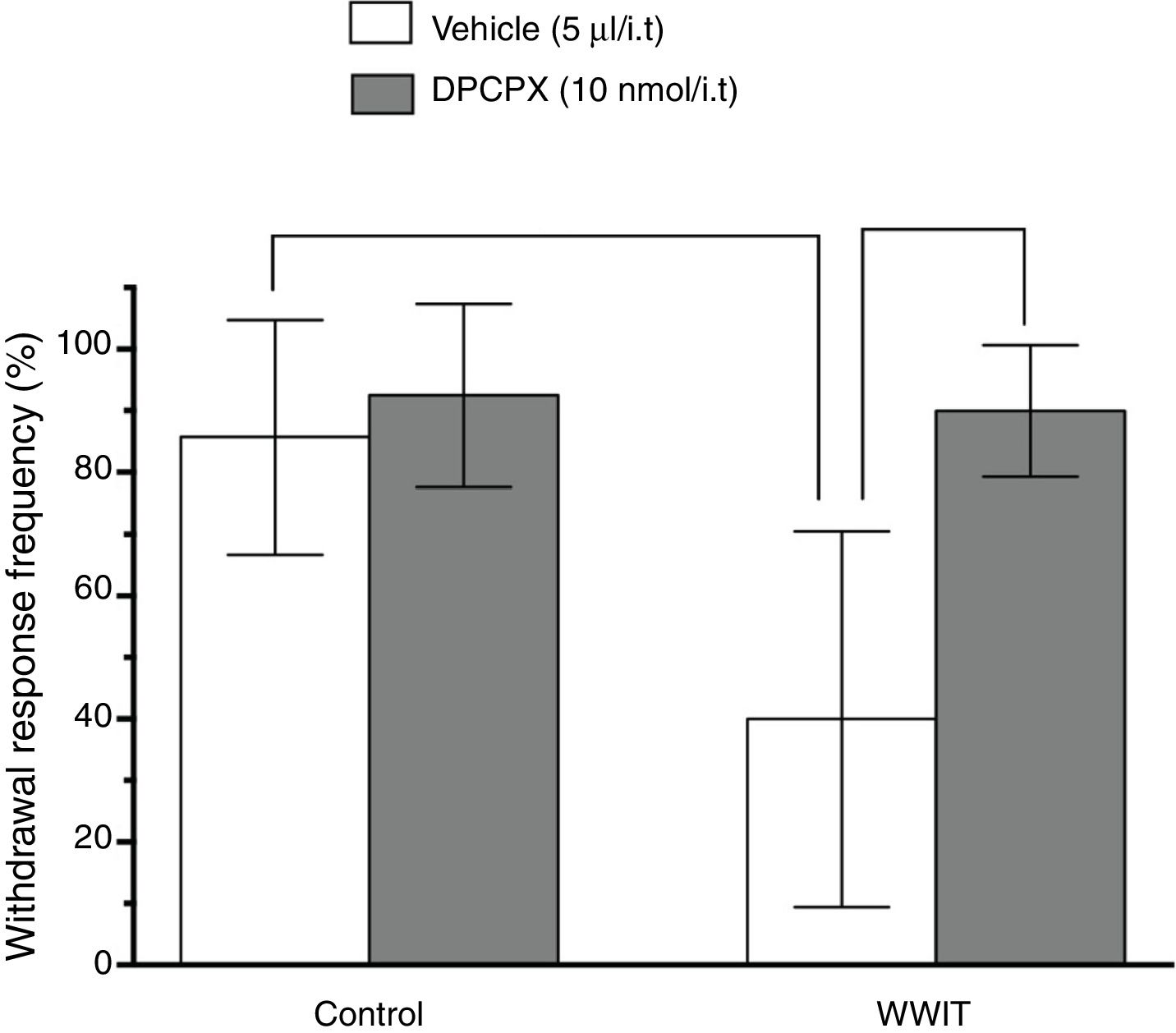
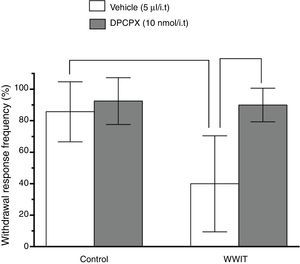
Effect of adenosine spinal receptors in the antihyperalgesic effect of WWIT. Mice’s CFA injected hindpaw after treatment in control water 25 °C immersion + Saline i.t. (white bar) or WWIT 35 °C (white bar). Half the mice received an i.t. injection of DPCPX prior to control water 25 °C immersion (gray bar) or WWIT 35 °C (gray bar). Results show the antihyperalgesic effect of WWIT 35 °C and how injection of DPCPX nullify that effect. Each point represents the mean of eight animals; vertical lines show SD. WWIT, warm water immersion therapy; **P < .01 and ###P < .001.
Two-way ANOVA revealed significant main effects of naloxone pretreatment [F(1,28) = 9.498; P < .004] and WWIT [F(1,28) = 27.64; P < .001], and a naloxone pretreatment x WWIT interaction [F(1,28) = 20.54; P < .001]. Post hoc analyses indicated that the pretreatment of mice with naloxone prevented (P < .001) the decrease withdrawal response frequency (pain-relieving effect) elicited by WWIT (Fig. 1).
In Fig. 2, Two-way ANOVA revealed significant main effects of AM281 pretreatment [F(1,26) = 11.26; P < .002] and WWIT [F(1,26) = 5.912; P < .022], and a AM281 pretreatment x WWIT interaction [F(1,26) = 14.39; P < .001]. Post hoc analyses indicated that the pretreatment of mice with AM281 prevented (P < .001) the decrease withdrawal response frequency (pain-relieving effect) caused by WWIT.
Two-way ANOVA revealed significant main effects of DPCPX pretreatment [F(1,26) = 15.46; P < .001] and WWIT [F(1,26) = 11.15; P < .002], and a DPCPX pretreatment x WWIT interaction [F(1,26) = 8.955; P < .006]. Post hoc analyses indicated that the pretreatment of mice with DPCPX prevented (P < .001) the decrease withdrawal response frequency (pain-relieving effect) produced by WWIT (Fig. 3).
DiscussionHere we demonstrated that WWIT produces antihyperalgesic effects in an experimental model of inflammatory pain and that this effect is mediated, at least in part, by a local spinal mechanism through activation of spinal opioid and A1 and CB1 receptors. Although we have initially demonstrated that WWIT produces antihyperalgesic effects in an experimental model of inflammatory pain and that this effect is mediated, at least in part, by a local peripheral mechanism, i.e., through activation of peripheral spinal opioid and A1 and CB2 receptors,11 the complete picture of the mechanisms involved in WWIT-induced antihyperalgesic effect still remains elusive.
It is well known that inflammatory pain may increase spinal cord expression and/or activity of voltage- and ligand-gated ion channels, peptide receptors, and neuroimmune factors, which then drive dorsal horn neuron hyperexcitability. The intensity and duration of central sensitization is determined by the net activity of local excitatory and inhibitory neurotransmitter systems, together with ongoing/evoked primary afferent activity and descending supraspinal control. Spinal endogenous inhibitory systems serve as opposing compensatory influences and are gaining recognition for their powerful capacity to restrain hyperalgesia. These include numerous G protein-coupled receptors (mu- and delta-opioid, alpha(2)-adrenergic, purinergic A1, neuropeptide Y1 and Y2, cannabinoid CB1 and CB2, muscarinic M2, gamma-amino-butyric acid type B, metabotropic glutamate type II-III, somatostatin.18
Opioid receptors are widely expressed in the central and peripheral nervous systems and in numerous nonneuronal tissues. Cannabinoids produce analgesic effects through activation of CB1R in the brain, spinal cord, and peripheral sites.19 It has been demonstrated that spinal administration of adenosine analogs produces analgesia in a broad range of nociceptive, inflammatory, and neuropathic pain tests.8 Spinal analgesia by adenosine is clearly mediated by A1R, as revealed by the use of selective agonists and antagonists.20 Analgesia induced by these three spinal receptors has recently been implicated in the effect of various physical therapy modalities. As an example, spinal opioid, CB1R, and A1R receptors have been implicated in antinociception induced by exercise21 and electroacupuncture22 in acute and chronic pain models. In this context, we investigated endogenous pain inhibitory systems, i.e., spinal opioid and A1 and CB1 receptors, in a mouse model of inflammatory pain. The results of the present study extend previous data by demonstrating, for the first time, that the antihyperalgesic effect of WWIT is mediated by spinal opioid, cannabinoid, and adenosine receptors.
Our group has previously shown that WWIT for 10 or 30 min, but not for 3 min, induces antihyperalgesia in the CFA-induced inflammatory pain model. In addition, we showed that the antihyperalgesic effect of 10-minute WWIT lasts for up to 1 h after treatment. Therefore, in the present study, we analyzed the mechanism of action 30 min after treatment.11 It has been suggested that the analgesic effect of WWIT might be simply due to increased temperature and the hydrostatic pressure of water on the skin.23 It has also been suggested that increasing skin temperature or deep tissue temperature would cause vasodilation in the vessels of damaged tissue and increase metabolism and blood flow, which in turn would result in increased removal of inflammatory compounds known to activate and sensitize primary afferent fibers. This would result in less input being transmitted to the spinal cord and higher brain centers and, thus, decreased perception of pain.24
Since inflammation decreases opioid receptors in the spinal cord, it may be hypothesized that the reduction of peripheral inflammation induced by WWIT could decrease primary afferent fibers sensitization (peripheral sensitization) which consequently reduces the release of neurotransmitters and/or neuromodulators and other inflammatory mediators in the spinal cord. Therefore WWIT, by modulating peripheral inflammation, could lead to increased expression of inhibitory receptors in the spinal cord and analgesia. This hypothesis is plausible since it is known that sensory nerve endings also express a number of receptors for neurotransmitters that can inhibit pain transmission. Many of these receptors were characterized initially in the dorsal spinal cord,5 some receptors that are synthesized in the cell body of dorsal root ganglia cells and transported centrally to reside presynaptically on primary afferent neurons are also transported peripherally.25 We raised the question of whether spinal opioid receptors could be involved in WWIT-induced analgesia. The results reported here indicate that the antihyperalgesic effect of WWIT is mediated through spinal opioid receptors as i.t. administration of naloxone prevented WWIT antihyperalgesic effect (Fig. 1).
Our data also demonstrates that activation of spinal CB1 receptors is directly related to the antihyperalgesic effect of WWIT. This conclusion is derived from the fact that i.t. pretreatment with AM281, a selective CB1R receptor antagonist, completely prevented the antihyperalgesic effect of WWIT. To corroborate these data, cannabinoid receptor agonists have been shown to inhibit inflammatory hyperalgesia in animal models more specifically, spinal cannabinoid receptor activation by the endogenous CB receptor agonist anandamide, has been demonstrated to inhibit inflammatory hyperalgesia when injected intrathecally.16 In addition, intrathecal administration of AM281 prevented anandamide's antihyperalgesic effect.16
Interestingly, morphine has been shown to induce endogenous adenosine release in synaptosomes obtained from the posterior spinal cord, and this release primarily originates adenosine.26 Morphine-induced adenosine release occurs via a bidirectional carrier that is present in capsaicin-sensitive neurons.27 Behavioral studies provide corroborative evidence of spinal adenosine release by opioids. Thus, spinal administration of methylxanthines inhibits morphine-induced spinal analgesia.26,28 Since we found that opioid receptors are involved in the antihyperalgesic effect of WWIT and given the fact that adenosine release may be induced by opioids, we also tested the hypothesis that A1R could mediate WWIT effects. Studies indicate that spinal analgesia caused by adenosine is mediated by A1R, as revealed by the use of selective agonists and antagonists.17,29 We have previously demonstrated that adenosine reduced mechanical hyperalgesia in a mice model of postoperative pain, and that pretreatment with DPCPX (a selective A1R antagonist) completely prevented this effect.17 Our results clearly demonstrate the involvement of spinal A1 receptors in WWIT-induced hyperalgesia, and from a clinical standpoint, the interpretation of these results suggests that caffeine consumption - which also antagonizes A1Rs - should be avoided, not to compromise the effectiveness of WWIT.
Taken together, these results confirm and largely extend previous literature data11 by demonstrating the involvement of spinal opioid and A1 and CB1 receptors in the antihyperalgesic effect of WWIT in a mouse model of inflammatory pain.
Conflict of interestThe authors declare no conflicts of interest.
The present study was supported by grants from Universidade do Sul de Santa Catarina - Curso de Medicina and Programa Unisul de Iniciação Científica (PUIC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-476454/2013-1 and CNPq-309407/2017-6), and Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC-3414/2012 and FAPESC-2019TR73), Brazil.