Osteoarthritis (OA) is a degenerative disease that induces peri-articular tissue degradation. OA induces an imbalance between synthesis and degradation of the extracellular matrix components in favor of catabolic events, promoting pathological remodeling and involving degradative enzymes, such as matrix metalloproteinases (MMPs).
ObjectiveThis study aimed to investigate the effects of 8-weeks resistance training (RT) on MMP-2 activity in the quadriceps tendon and patellar tendon in an OA model.
MethodsTwenty-four Wistar rats were randomly divided into six groups: Control, Exercise, Sham, Sham with Exercise, OA, and OA with Exercise (OAE). The OA model was performed by anterior cruciate ligament transection surgery on the left knee. The 8-week RT consisted of climbing a 1.1-m vertical ladder three times per week with progressive weights secured to the animals’ tails. MMP-2 activity was analyzed by zymography.
ResultsThe OAE group displayed lower pro, intermediate, and active MMP-2 activity in the quadriceps tendon compared with the OA group (p<0.05). For the patellar tendon, there was no significant difference between the OAE group compared with the other groups (p>0.05) for pro, intermediate, and active MMP-2 activity. Moreover, MMP-2 activity differed between tissues, the OA and OAE groups presented lower pro, intermediate, and active MMP-2 activity in the quadriceps tendon compared to the patellar tendon.
ConclusionRT induced down-regulated MMP-2 activity in the quadriceps tendon. RT is a potential therapeutic approach to minimize the deleterious effects of extracellular matrix degeneration.
Osteoarthritis (OA) is a degenerative disease characterized by progressive compromised joint physiological function, involving bones and synovial joints.1,2 Previous studies have shown that a joint affected by OA displays progressive degeneration of articular cartilage,3 formation of osteophytes,4 and fluid synovial inflammation.5 The deleterious molecular effects include cellular stress6 and secretion of catabolic molecules, accompanied by a high degradation of extracellular matrix (ECM).7 The ECM provides mechanical properties and maintenance of the functional integrity of the joint.8
OA induces an imbalance between synthesis and degradation of ECM components in favor of catabolic events promoting pathological tissue remodeling which can eventually lead to its breakdown.7 This ECM disorder involves an increase of matrix metalloproteinases (MMPs) activity. The MMPs constitute a large and heterogeneous family of zinc-dependent endopeptidases that modulate ECM functions by cleavage of internal peptide bonds, degrading their constituents, such as proteoglycans, collagen, fibronectin, and laminin.9 Previous studies have shown that MMP-2 (Gelatinase A) is one of the major enzymes involved in OA occurrence and progression. MMP-2 activity is enhanced in osteoarthritic cartilage and peri-articular tissue, suggesting that this type of MMP is a relevant biological target in OA pathogenesis.10 It has been reported that MMP-2 plays a crucial role in joint destruction because they perform regulation of morphogenesis in connective tissue.7,10
Pharmaceutical therapy is the most commonly used treatment for OA,11 however, the deleterious effects are mostly neglected.11 Minimally invasive interventions including exercise can be as effective as drugs in providing symptom relief, without serious side effects.11,12 In a systematic review and meta-analysis Li et al.11 demonstrated that resistance training (RT) is a viable alternative to improving quality of life in patients with knee OA, alleviating stiffness and improving muscle strength and physical function. There is evidence that RT (i.e. four weeks, four times per week) can reduce MMP-3 expression, cytokines in synovial fluid, and improve clinical symptoms in patients with OA.12 Furthermore, a combination of RT and aerobic exercise of moderate intensity has shown to have a protective effect on articular cartilage and to improve neuromuscular control and glycosaminoglycans content in patients with a high risk of OA.13 In animal models, Milares et al.14 showed a decrease in MMP-13 expression induced by RT (eight weeks, three times per week) associated with a low-level laser on the nucleus of chondrocytes in a rat model of OA, which indicates that RT exerts significant synergistic effects on recovery from cartilage degeneration.
Although the molecular alterations induced by exercise training have been widely described in OA, the link between the activity of MMP-2 in peri-articular structures with RT programs remains to be determined. This information is essential, as OA also promotes instability and even progressive degeneration of the peri-joint and consequently MMP-2 is abundantly expressed.15 MMP-2 is involved in the collagen breakdown and its activity is an indicator of OA occurrence and progression.7 Another factor that should be considered is that the quadriceps tendon is an active structure, which has a relationship with skeletal muscle, possibly favoring recruitment of molecules by this tissue.16 On the other hand, patellar tendon is a more passive structure when compared with quadriceps tendon, because they are more distant from the skeletal muscle and devoid of dynamic responses in microcirculation, which consequently characterizes a metabolically less active portion.17 Nevertheless, it is still unknown which of these structures can benefit from RT.
The purpose of this study was to investigate the effects of 8 weeks of RT on MMP-2 activity in the quadriceps and patellar tendons in a rat model of OA. We hypothesized that RT would promote a decrease in MMP-2 activity in the quadriceps and patellar tendons, minimizing the deleterious effects of OA.
MethodsStudy designThe sample size calculation for the present study was based on a priori power analysis which indicated the need to include 36 male rats (Wistar) obtained from the Central Animal Laboratory of the Universidade Federal de São Carlos. The animals were housed in plastic cages (maximum three rats per cage) in an animal room under controlled environment conditions (12h cycle of light/dark), with free access to standard food (Purina®, Descalvado, São Paulo, Brazil) and water. The experiment was conducted in accordance with international ethical recommendations (National Research Council, 1996) and the project was approved by the Animal Experimentation Ethics Committee of the Universidade Federal de São Carlos (UFSCar), São Carlos, SP, Brazil (CEA/021/2010).
The animals were randomly distributed into six groups: control (C, n=6), exercise (E, n=6), sham without exercise (S, n=6), sham with exercise (SE, n=6), OA without exercise (OA, n=6), and OA with exercise (OAE, n=6). The control group did not perform RT while the exercise groups performed RT consisting of climbing a 1.1-m vertical ladder with weights secured to the tail. Sham group (i.e., placebo surgery) was a faked surgical intervention that omits the step thought to be therapeutically necessary. The animal model of OA used was performed by anterior cruciate ligament transection (ACLt). Two weeks after the ACLt, the E, OAE, and SE groups started the RT program. The animals were weighed in grams using a digital scale (Filizola®, São Paulo, Brazil) before and after RT.
Experimental procedureAnimal model of OA (ACLt)Rats submitted to the ACLt were anesthetized with Ketamine (95mg/kg) and Xylazine (12mg/kg) intraperitoneally before the procedure. Prior to surgery, the left knee of the animals was shaved and a parapatellar skin incision (1cm) was performed first on the medial side, followed by the medial side of the patellar tendon. The rear paw of the left knee joint was flexed to expose the anterior cruciate ligament (ACL) as far as possible. Then, the patella was dislocated laterally to expose the ACL and provide access to perform the ACLt. Subsequently, the patella was relocated in its proper location, and the tissues sutured according to the recommendations from Galois et al.18
A positive anterior drawer test was used to validate the complete transection of the ligament. No antibiotic or anti-inflammatory medication was administered to the animals. The Sham group undergoing knee arthrotomy without ACLt was included to characterize the experimental model.
Resistance training periodThe rats were familiarized with the RT protocol by climbing a vertical ladder (1.1×0.18m, 2-cm grid, 80° incline) with no weight on the load apparatus for two non-consecutive days. The load apparatus was fixed to the tail by a wrapping of the proximal portion with adhesive tape (Micropore®). At the top of the ladder, the animals reached a cage (20×20×20cm). The RT protocol was adapted from the study by Hornberger and Farrar19 according to the needs of the current research. The training procedures have also been described in other studies using animal models.9,20,21
Determination of maximum carrying capacityOn the first day of the protocol, each animal was evaluated to establish its maximum carrying load (CL). The CL consisted of 4–8 ladder climbs with progressively heavier loads interspersed by a 120 second interval between each attempt. The initial climb was performed with 50% of the animal's body mass (±150g). Upon successful completion of this load, additional 30-g weight was added to the load apparatus. The highest load that the animal successfully carried through the entire length of the ladder was considered the maximal carrying capacity for that training session. Failure was determined when the animal could not progress up the ladder after three successive stimuli to the tail.
Resistance exercise sessionsThe animals performed 10 climbing sessions with a 30-s interval between each climb, three sessions per week on alternate days, for a total period of 8 weeks. The RT protocol was progressive in the following evolution: 1st and 2nd weeks 50% CL, 3rd and 4th weeks 75% CL, 5th and 6th weeks 90% CL, 7th and 8th weeks 100% CL. The total training volume (total ladder climbs completed×load) was adapted from the work by Sousa Neto et al.9
Euthanasia and tissue preparationThe animals were sacrificed 48h after the end of the experiment using an intraperitoneal injection of xylazine (12mg/kg of body weight) and ketamine (95mg/kg of body weight). The rats were placed on a surgical table in the supine position with the legs joined and extended, for removal of the quadriceps and patellar tendons. The tissues were frozen in liquid nitrogen and stored in a freezer at −80°C.
Experimental outcomesThe quadriceps and patellar tendons were dissected and immediately washed with saline. The samples were weighed and frozen in liquid nitrogen and stored in a freezer at −84°C for subsequent biochemical analysis. The tissue samples were treated as described elsewhere for extracts.22 Frozen tissue (25mg) was incubated in 2mL of extraction buffer (10mM–1 cacodylic acid (pH 5.0), 0.15M–1 NaCl, 1μM–1 ZnCl2, 20mM–1 CaCl2, 1.5mM–1 NaN3, 0.01% Triton X-100 (v/v)) at 4°C overnight with continuous mixing. After this period, the solution was centrifuged for 20min (13000×g at 4°C). Protein concentrations were measured with a BCA protein assay kit (Thermo Scientific, USA). Samples of 6 animals from each group were evaluated to guarantee the precision and linearity of the analysis, and each sample was normalized for the total amount of protein (25mg). The samples were resolved by electrophoresis in a polyacrylamide gel containing SDS 10% and gelatin at a final concentration of 1mg/mL. After electrophoresis, the gels were washed twice in 2.5% Triton X-100 to remove SDS and incubated in substrate buffer (50mM–1 Tris–HCl (pH 8.0), 5mM–1 CaCl2 and 0.02% NaN3) at 37°C for 20h. The gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad) for 1.5hours and destained with acetic acid, methanol, and water. The gels were photographed with a digital camera (Canon G6 PowerShot 7.1 megapixels). Densitometric quantitative analysis of the MMP-2 protein bands seen in the zymography gels was performed using the software Gene Tools version 3.06 (Syngene, Cambridge, UK). Isoforms bands were identified via standard techniques using molecular weight criteria according to previous studies.22,23
Statistical methodsThe results are expressed as means±standard deviation. The Shapiro–Wilk test was applied to verify normal distribution of data and the Levene's test was used to analyze the homogeneity of variance. Body mass and total volume training of the experimental groups presented normal distribution and homogeneity. A paired-samples Student t-test identified differences between the initial and final values in the same group. The one-way ANOVA test was used for comparisons of maximum load capacity and total volume training between trained groups. The non-parametric Kruskal–Wallis test was used to compare MMP-2 activity. Appropriate pairwise comparisons were performed using Dunn's procedure with the Bonferroni correction for multiple comparisons. Furthermore, for comparisons between tissues, the Mann–Whitney U test was applied. The level of significance was set at alpha ≤0.05. All analyses were conducted with statistical package for social sciences (SPSS, Inc., v. 21.0; IBM Corporation, Armonk, NY, USA).
ResultsBody mass and load capacity of experimental groupsAll 36 rats completed the experiments and were included in the analysis. Six animals from each group showed a significant increase in body mass after the experimental period, with no differences between groups. All trained groups (i.e. groups that received exercises) increased their maximal load capacity compared to baseline, but no differences between trained groups were found. In addition, no significant differences were observed between trained groups with respect to total training volume (Table 1).
Body mass and total training volume of experimental groups.
| C | E | S | SE | OA | OAE | |
|---|---|---|---|---|---|---|
| Initial body mass (g) | 265.20±22.16 | 265.70±12.71 | 277.30±7.76 | 264.00±27.11 | 270.80±26.78 | 261.50±9.18 |
| Final body mass (g) | 400.00±30.85a | 404.70±19.47a | 432.50±30.45a | 453.80±31.74a | 452.50±44.31a | 419.00±2.81a |
| Initial maximal load (g) | 195.00±13.89 | 200.60±11.16 | 198.80±13.30 | |||
| Final maximal load (g) | 390.00±27.77b | 401.30±22.32b | 397.50±26.59b | |||
| Total volume training (g) | 3900.00±277.70 | 4013.00±223.20 | 3975.00±265.90 |
The data are mean±standard deviation. The experimental groups: control (C, n=6), exercise (E, n=6), sham without exercise (S, n=6), sham with exercise (SE, n=6), osteoarthritis without exercise (OA, n=6), and osteoarthritis with exercise (OAE, n=6). Total training volume=total ladder climbs completed×load. Statistically significant differences compared to:
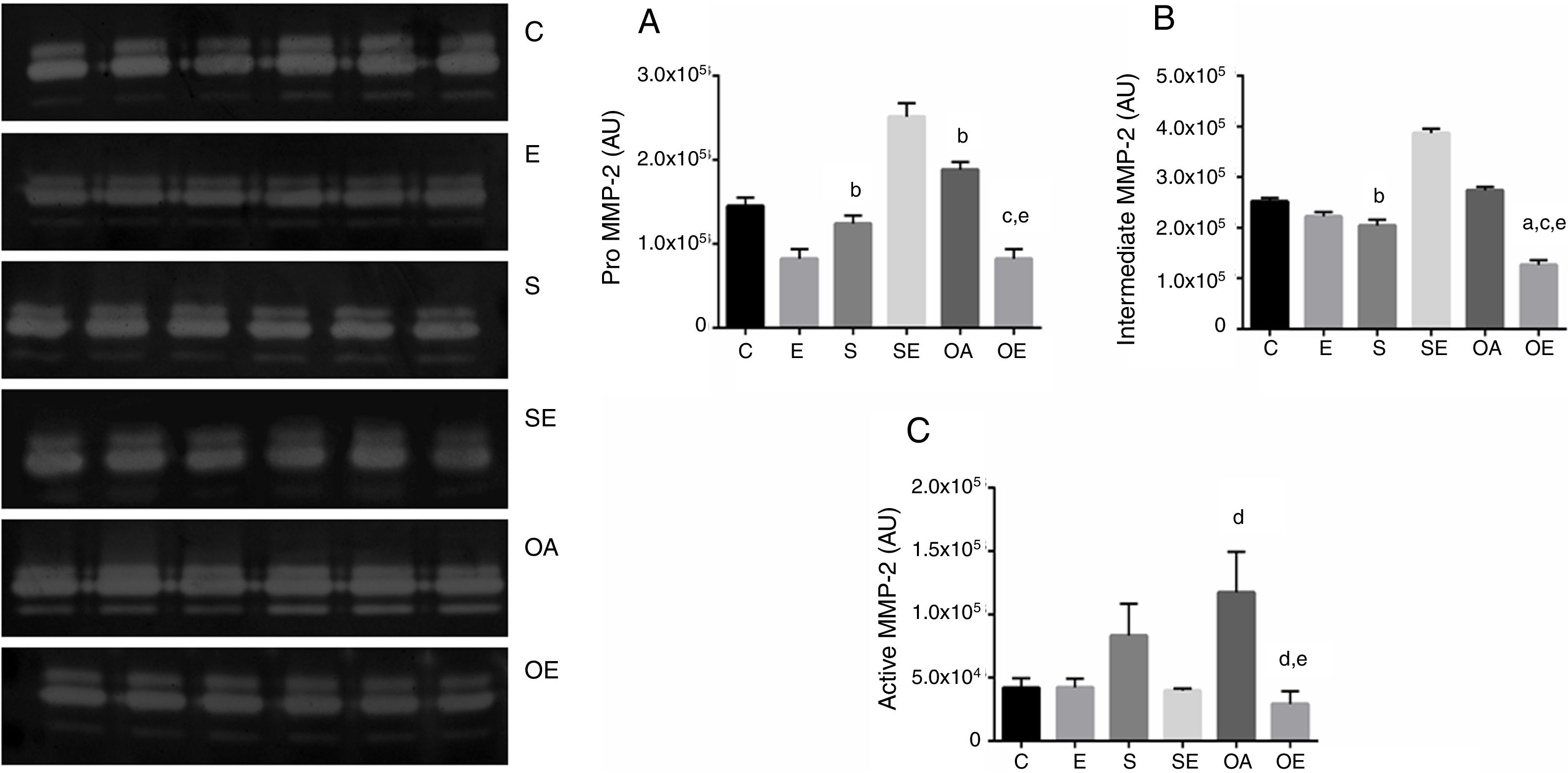
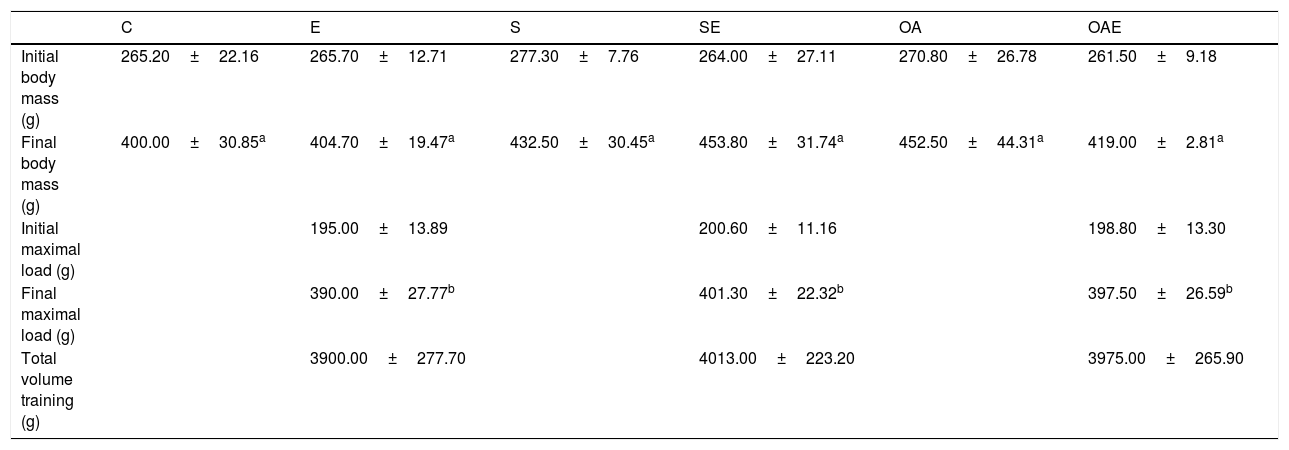
The pro MMP-2 activity in the quadriceps tendon was higher in the S and OA groups when compared with the E group (p=0.01). The OAE group was lower compared to the S and OA groups (p=0.001; Fig. 1A).
Optical densitometry of zymography bands of MMP-2 in arbitrary units (AU) in the quadriceps tendon. The data are mean±standard deviation. (A) Pro MMP-2 (72kDa), (B) intermediate MMP-2 (64kDa), (C) active MMP-2 (64kDa). The experimental groups: control (C, n=6), exercise (E, n=6), sham without exercise (S, n=6), sham with exercise (SE, n=6), osteoarthritis without exercise (OA, n=6), and osteoarthritis with exercise (OAE, n=6). Statistically significant differences compared to: aC; bE, cS, dSE, eOA, p<0.05.
There was lower intermediate MMP-2 activity in S when compared with the E group (p=0.03). Furthermore, there was lower activity of this isoform in OAE when compared with the C, S, and OA groups (p=0.001; Fig. 1B).
With respect to active MMP-2 activity, the OA group showed higher activity when compared with the SE group (p=0.02). The OAE group showed lower activity compared to SE and OA groups (p=0.001; Fig. 1C). No MMP-9 activity was detected by zymography.
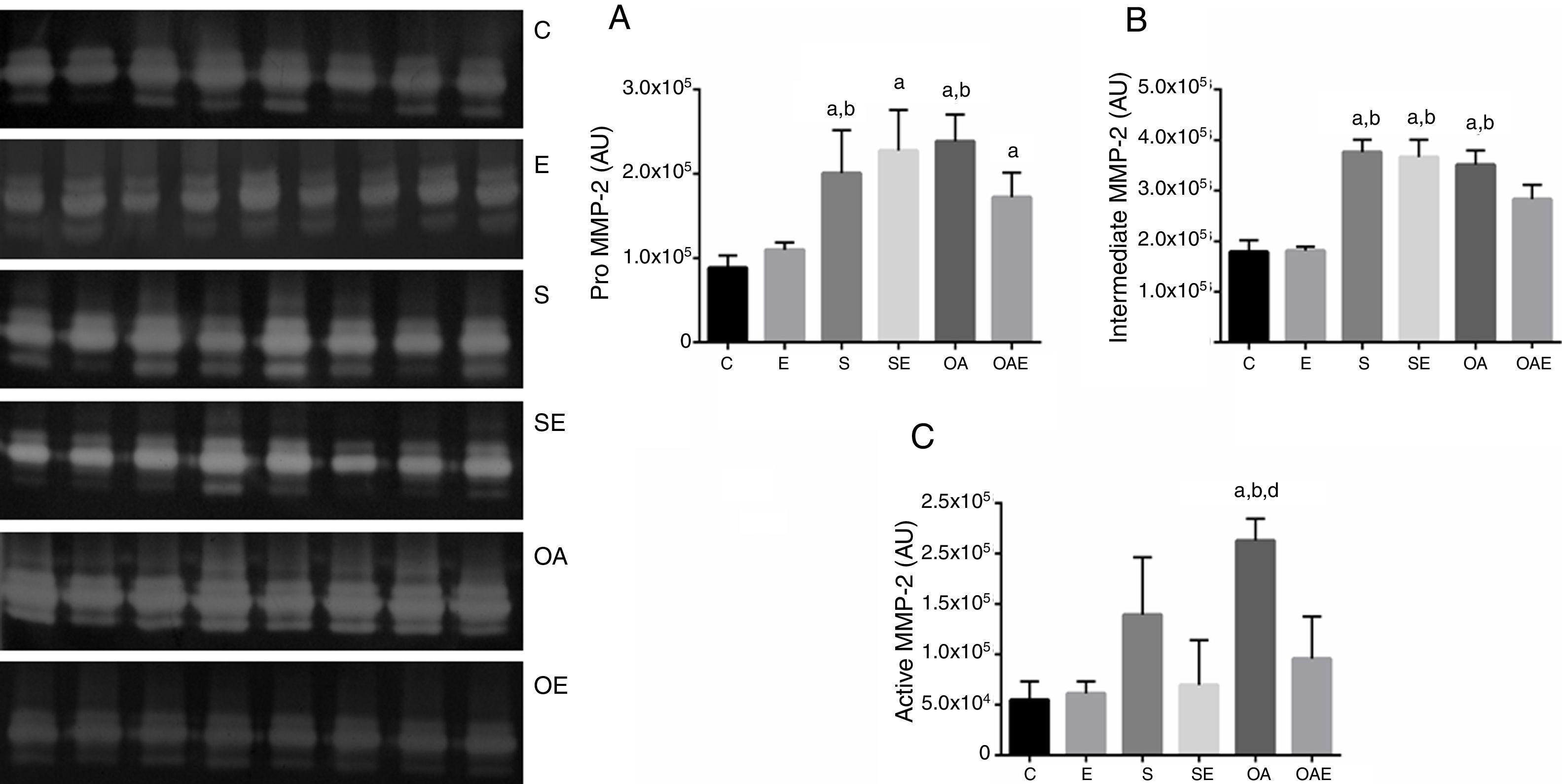
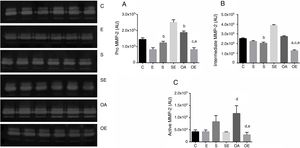
Effects of RT on MMP-2 activity in patellar tendonThe pro MMP-2 activity in patellar tendon was higher in S and OA groups when compared with the C and E groups (p=0.01). In addition, The SE and OE groups also had higher pro MMP-2 activity when compared to C group (p=0.01) (Fig. 2A).
Optical densitometry of zymographic bands of MMP-2 in arbitrary units in the patellar tendon. The data are mean±standard deviation. (A) Pro MMP-2 (72kDa), (B) intermediate MMP-2 (64kDa), (C) active MMP-2 (64kDa). The experimental groups were represented control (C, n=6), exercise (E, n=6), sham without exercise (S, n=6), sham with exercise (SE, n=6), osteoarthritis without exercise (OA, n=6), and osteoarthritis with exercise (OAE, n=6). Statistically significant differences compared to: aC; bE, dSE, p<0.05.
There was higher intermediate MMP-2 activity in S, SE, and OA groups when compared with the C and E groups (p=0.01; Fig. 2B). Regarding active MMP-2 activity, the OA group showed the highest values when compared to the C, E, and SE groups (p=0.001; Fig. 2C). No MMP-9 activity was detected by zymography.
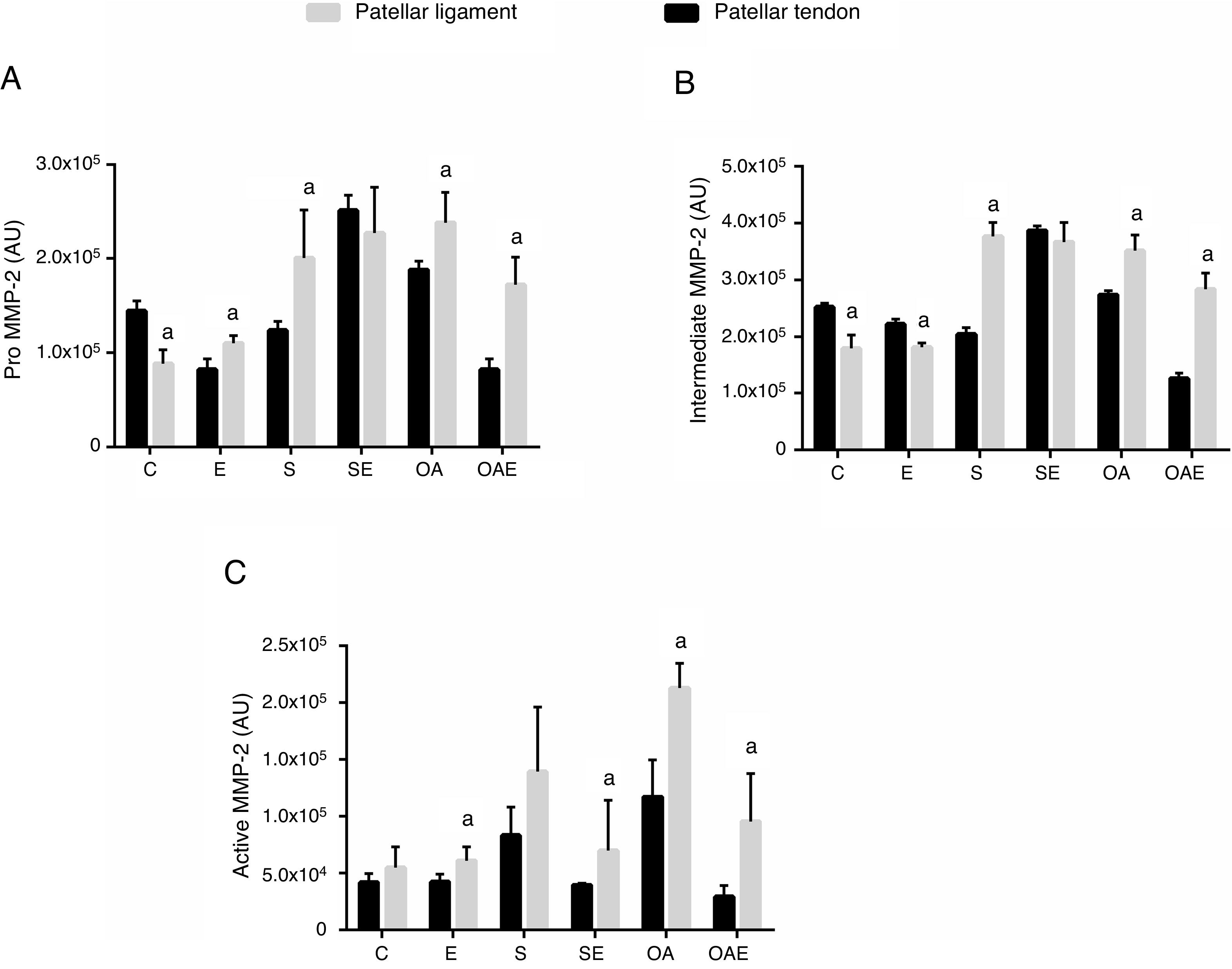
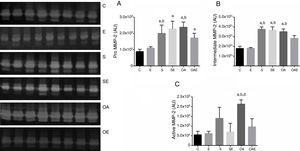
Comparison of MMP-2 activity between quadriceps and patellar tendonsFor the pro MMP-2, higher activity in the patellar tendon was found in the E, S, OA, and OAE groups (p=0.01) when compared to the quadriceps tendon. In contrast, lower activity was found in the patellar tendon for the C group (p=0.001; Fig. 3A).
Optical densitometry of zymographic bands of MMP-2 in arbitrary units between the quadriceps and patellar tendons. The data are mean±standard deviation. (A) Pro MMP-2 (72kDa), (B) intermediate MMP-2 (64kDa), (C) active MMP-2 (64kDa). The experimental groups were represented control (C, n=6), exercise (E, n=6), sham without exercise (S, n=6), sham with exercise (SE, n=6), osteoarthritis without exercise (OA, n=6), and osteoarthritis with exercise (OAE, n=6). a=statistically significant differences between quadriceps and patellar tendons, p<0.05.
For the intermediate MMP-2, higher activity was observed in the patellar tendon in the S, OA, and OAE groups (p=0.001). However, lower activity was observed in the patellar tendon for the C and E groups (p=0.001; Fig. 3B).
Finally, regarding active mmp-2, differences between quadriceps tendon versus patellar tendon were found for the E, SE, OA, and OAE groups, indicating higher activity in the patellar tendon when compared with the quadriceps tendon (p=0.01; Fig. 3C).
DiscussionThe present study investigated the effects of 8 weeks of RT on MMP-2 activity in the quadriceps and patellar tendons in a rat model of OA. RT lead to lower MMP-2 activity in the quadriceps tendon, minimizing the deleterious effects of OA. This finding partially supports the hypothesis of this study that RT could have a protective effect for the development of degenerative OA through inhibition of MMP-2 mechanisms. However, RT did not show the same findings for the patellar tendon. The novel finding of this study was that MMP-2 activity differed between the quadriceps and patellar tendons, demonstrating higher activity in the patellar tendon when compared to the quadriceps tendon.
The peri-articular structures represent a musculoskeletal system component which contains highly abundant ECMs (around 80%), essential for stabilization and structural organization of joints.17,24 The tendon plays an important role in the transfer of force between the muscles and bones, providing mechanical support.17,24 In contrast, ligaments have the function of connecting bones and protecting and stabilizing the joints to avoid unnecessary displacements.17 Most significantly, the current study found that RT induced a closed interaction between mechanical signaling and biochemical changes in the ECM required in peri-articular structures. The local decrease in MMP-2 activity is important to the maintenance of physiological functions and tissue homeostasis and must still be considered as a potential mechanism in the protection of ECM degeneration.23
On the other hand, studies have shown the influence of training on ECM adaptations in knee OA. These studies reported on large scale only the collagenases adaptations14,25 and neglected the importance of gelatinases such as MMP-2. It has been reported that MMP-2 degrades collagens type IV, V, VII, and XI acting synergistically with collagenases in the cleavage of collagens.26 Furthermore, there is now persuasive evidence that MMP-2 acts together with MMP-1 in the degradation and denaturation of type II collagen and aggrecan core protein in cartilage.27,28 Thus, this work represents an essential first step in understanding MMP-2 activity as a key biochemical marker in peri-articular structures in a rat model of OA.
The rationale to use the RT model in this study was based on previous studies demonstrating that several types of exercise including cardiovascular conditioning, and balance training can decrease pain symptoms and improve functional aspects in patients with OA.11,13,14,18,25,29 In addition, recent studies showed that training plays an important role in the anabolic effects on cartilage, decreases inflammatory mediators and promotes down-regulation of catabolic enzymes in ECM.14,25
To our knowledge, this is the first investigation to analyze the effects of RT on MMP-2 activity in peri-joints in an OA rat model. However, previous studies have demonstrated that training can protect other structures of the knee joint.14,25 For example, it was demonstrated that aerobic exercise training is helpful to facilitate a better pattern of tissue organization, with less fibrillation and irregularities along the articular surface, in rat with knee OA.25 Additionally, the aerobic exercise training group showed reduced expression of IL-1β and MMP-13 when compared to the control group. Similarly, Milares et al.14 showed a decrease in MMP-13 expression induced by aquatic exercise (8 weeks, three times per week) on the nucleus of chondrocytes. In this way, these investigations contribute to explaining the beneficial effect that exercise has against degradation of the knee joint.
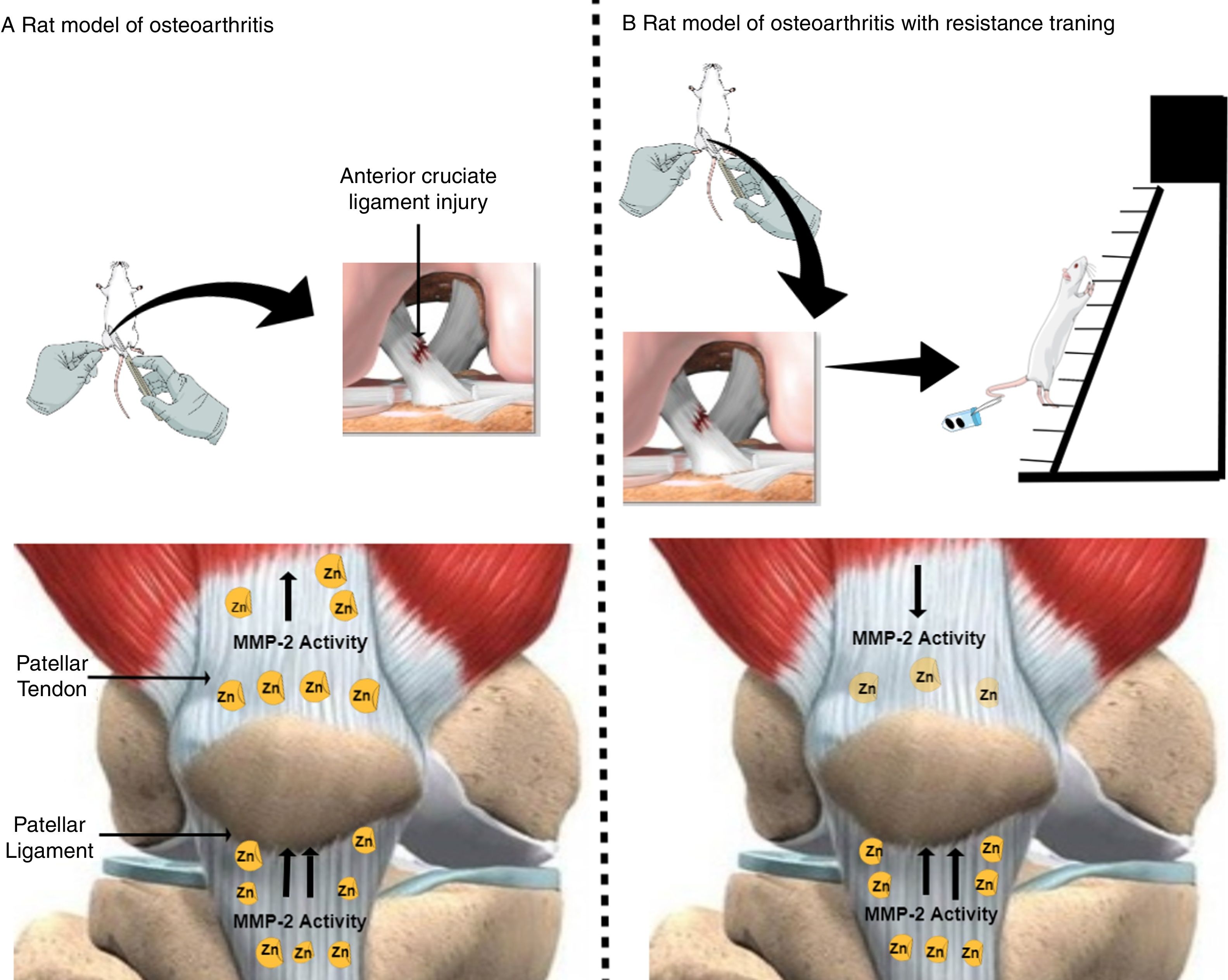
An exciting result of our study was that RT promotes decreased MMP-2 activity in the quadriceps tendon, but not in the patellar tendon (Fig. 4). We might speculate that as the quadriceps tendon is an active structure,16 which has an intimate relationship with skeletal muscle, it favors recruitment of additional molecules by the muscle for ECM remodeling. However, patellar tendons are passive structures when compared with quadriceps tendons17 because they are more distant from skeletal muscle and thus devoid of circulation and nutrients, which could explain higher MMP-2 activity in the patellar tendon when compared to the quadriceps tendon. Therefore, a comprehensive assessment of peri-articular structure and function are required, in addition to measures of MMP-2 activity. Treatments based on exploitation of these interactions may lead to improved health in OA.
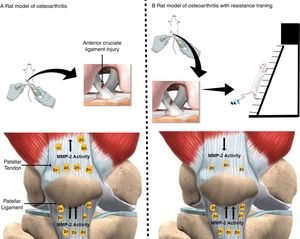
Effects of osteoarthritis and resistance training on matrix metalloproteinase-2 activity in the quadriceps and patellar tendon of rats. (A) Rat model of osteoarthritis (exposed to anterior cruciate ligament injury). (B) Rat model of osteoarthritis with resistance training (consisted of climbing a vertical ladder with weights secured to the tails). Yellow oval represents matrix metalloproteinase-2 (MMP-2). (↑) Up-regulated MMP-2 activity and (↓) down-regulated MMP-2 activity. The figure was created in the Mind the Graph platform (www.mindthegraph.com). The image from Mind the Graph, http://mindthegraph.com/, is licensed under the Attribution-Share Alike 4.0 International license.
Another significant finding of the present study was that the OA and OE groups presented lower levels of MMP-2 activity isoforms in the quadriceps tendon when compared with the patellar tendon. Peri-joint structures such as the quadriceps and patellar tendons possess a limited ability for self-repair in response to degenerative articular disease.17 Accumulated evidence from animal and human studies suggests that patellar tendons are injured more often than any other joint structures,30–32 which can result in significant disability for the patient.
It has been shown that an interaction of molecules occurs between tendon and skeletal muscle such as growth factors and migration/proliferation of proteins to modulate the morphogenesis, which can contribute to minimizing the deleterious effects of OA in the quadriceps tendon.16 Furthermore, these authors reported that muscle fiber also contributes to the tendon matrix by secreting ECM components into its surroundings and some of these components overlap with those of the tendon.16 On the other hand, the ligament of the knee may be an insufficient carrier of growth factors and mesenchymal stem cells, which hinders remodeling in the repair site.
Some limitations of the present study should be highlighted, such as the lack of analysis of inflammatory cytokines, gene expression levels, and investigation of morphological properties of ECM in the quadriceps and patellar tendons. These interactions should be investigated to clarify the involved mechanisms. In addition, peri-articular function is a relevant approach to be considered in future studies.
ConclusionRT induced different responses of MMP-2 activity in the quadriceps and patellar tendons in a rat model of OA. The RT led to a reduction in MMP-2 activity in the quadriceps tendon of rats with knee OA, but not in the patellar tendon.
Conflicts of interestThe authors declare no conflicts of interest.
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. The authors also are grateful for the financial support provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) from Brazil.