Noninvasive ventilation (NIV) is used in patients with post-traumatic respiratory failure, however, previous reviews indicate that its effects remain uncertain due to limited data.
ObjectiveTo evaluate the effects of NIV on mortality, complications, infection, and intensive care unit (ICU) and hospital length of stay in adult patients with blunt chest trauma.
MethodsMEDLINE (PubMed), EMBASE, Cochrane CENTRAL, Web of Science, PEDro and Scielo were searched from inception to February 2024. Randomized clinical trials comparing NIV with invasive mechanical ventilation (IMV) or oxygen therapy for respiratory failure in adults with blunt chest trauma were included. The outcomes analyzed were mortality, complications, infections, and length of stay in the ICU. Risk of bias was assessed using the RoB 2.0. Certainty of evidence was evaluated according to Grading of Recommendation, Assessment, Development and Evaluation (GRADE).
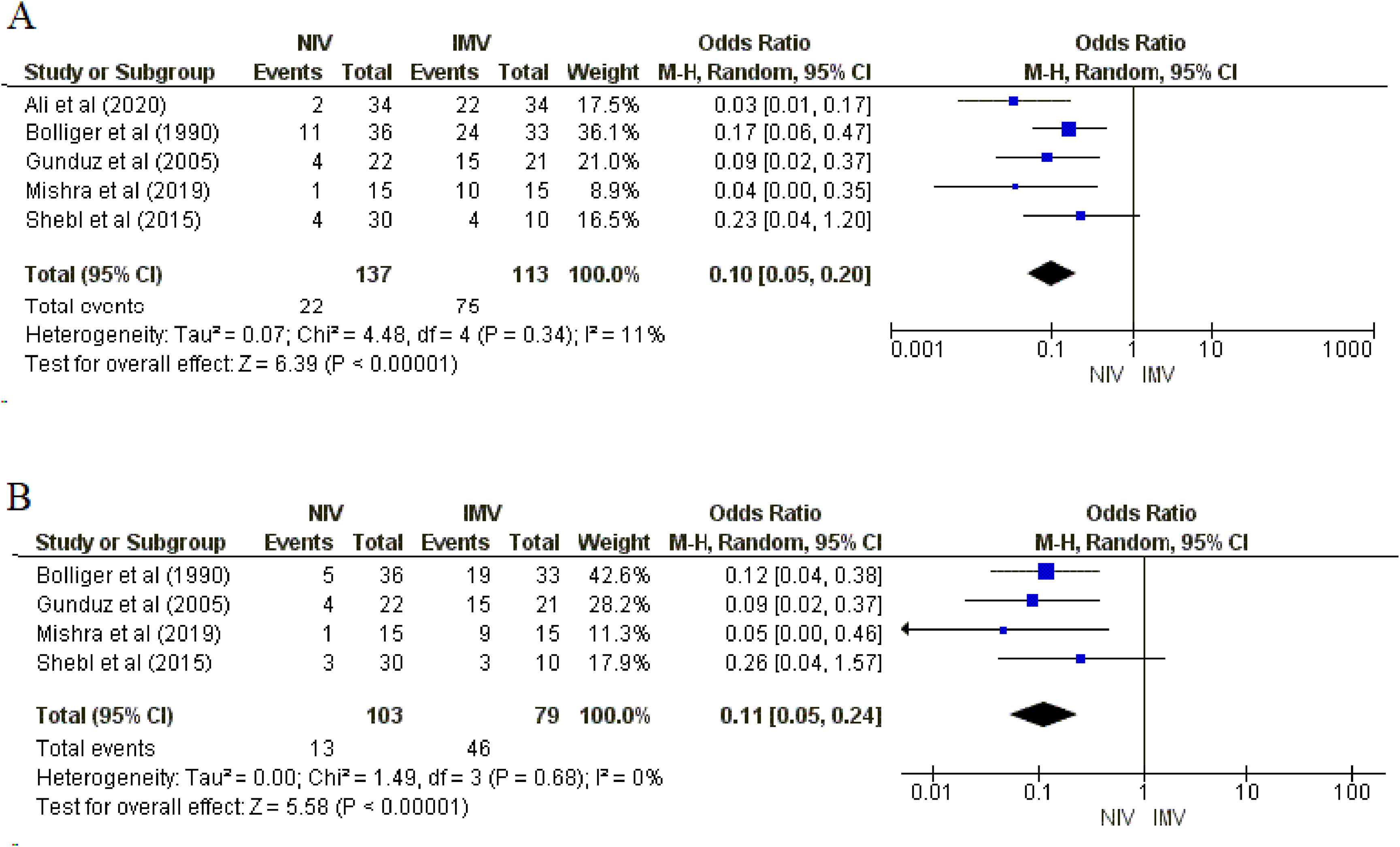
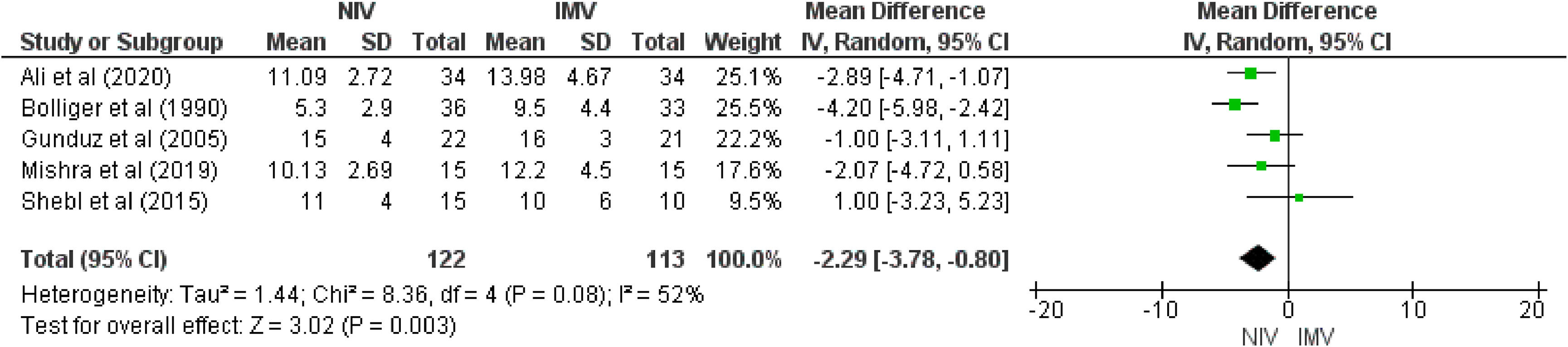
ResultsSix studies (300 patients) were included. Meta-analysis showed that NIV likely reduces mortality (5 studies, Odds Ratio [OR] = 0.15; 95%CI 0.06, 0.37; moderate certainty of evidence) compared with IMV. NIV may reduce complications (5 studies; OR= 0.10, 95%CI 0.05, 0.20) and infections (4 studies; OR = 0.11, 95%CI: 0.05, 0.24) (moderate certainty of evidence for both). For length of stay in the ICU, NIV may reduce the number of days in the ICU (5 studies; mean difference = -2.29 days; 95%CI -3.78, -0.80; low certainty of evidence) compared with IMV.
ConclusionNIV has the potential to reduce mortality, complications, infections, and ICU length of stay, compared to IMV in patients with respiratory failure after blunt chest trauma.
Chest trauma is a major contributor to significant mortality and morbidity worldwide, causing or contributing to more than a quarter of trauma-related deaths.1 This can be categorized into two types: penetrating and blunt. In blunt injuries, a direct impact to the anterior chest wall damages the underlying organs and structures without compromising the skin's integrity.2,3 Chest wall and lung injuries caused by blunt chest trauma are associated with the development of acute pulmonary failure in up to 20 % of patients.4
Respiratory failure is associated with pulmonary contusion, respiratory inefficiency associated with pain and reduced vital capacity, early or late post-traumatic pneumonia, pneumothorax, hemothorax, and empyema.5 Post-traumatic respiratory failure within 72 h is associated with a high mortality rate and, as a result, patients require rapid and efficient ventilatory management.6,7
Endotracheal intubation and invasive ventilation are performed in 23–75 % of patients with chest trauma; however, these procedures are associated with increased complications and prolonged ventilation and hospital stay.8,9 The need for invasive ventilatory support is determined by the patient's general clinical severity, defined by changes in physiological parameters and the severity of injuries.10
The Advanced Trauma Life Support (ATLS®) for the treatment of traumatic lung contusion evaluated the role of noninvasive ventilatory support and concluded that patients with severe traumatic lung contusions can be safely treated with noninvasive ventilatory support (Continuous Positive Airway Pressure [CPAP] or Bilevel Positive Airway Pressure [BiPAP]).11 Noninvasive ventilation (NIV) is recommended in cases of thoracic trauma associated with acute respiratory failure,12 following criteria such as moderate to severe respiratory distress, tachypnea (respiratory rate >25 breaths/min), use of accessory muscles or abdominal paradox, blood gas derangements (pH 〈 7.35, PaCO2 〉 45 mmHg), and impaired oxygenation (PaO2/FiO2 < 300 or SpO2 < 92 % with FiO2 of 0.5).13 However, there are contraindications to the use of NIV, including cardiac or respiratory arrest, nonrespiratory organ failure (severe encephalopathy, severe gastrointestinal bleeding, hemodynamic instability), facial trauma, upper-airway obstruction, inability to protect the airway, and/or high risk of aspiration.14 Furthermore, although the risk of worsening pneumothorax under NIV is low, careful monitoring in patients with undrained pneumothorax is essential.15
The greatest evidence for the use of NIV in acute respiratory failure has been reported in patients with cardiogenic pulmonary edema and Chronic Obstructive Pulmonary Disease exacerbation, with a reduced risk of intubation and hospital mortality.12 In patients with chest trauma, some reports have shown that NIV reduced mortality9,16 and intensive care unit (ICU) length of stay16 compared to invasive mechanical ventilation (IMV) and reduced intubation rate17 compared with oxygen therapy.
Likewise, the systematic review by Chimuello et al.,18 which included patients with chest trauma but also with respiratory failure due to other causes, indicated the early use of NIV reduces mortality and intubation rate without increasing complications. Contrary to these results, a systematic review published more than 10 years ago, which included only three randomized clinical trials, demonstrated that NIV did not present a significant reduction in the risk of mortality.19
In addition, another systematic review20 reported that there is no apparent benefit of NIV in preventing intubation in patients with respiratory decompensation. The same authors also mentioned that the use of NIV in patients with chest trauma who have associated acute lung injury with respiratory distress remained controversial due to the lack of good quality data. It is noteworthy that the majority of studies included in these previous reviews were observational and cohort studies, largely of low quality.
Thus, the present review proposes to update the literature, because the previous reviews are from more than 10 years ago and included studies with designs and biases that can affect the quality of the evidence. The results of this study will provide evidence for physical therapy practice in respiratory care and rehabilitation, contributing to optimizing clinical outcomes and improving recovery strategies for patients with blunt chest trauma. Hence, this systematic review and meta-analysis of randomized clinical trials aimed to evaluate the effects of NIV compared to IMV or oxygen therapy on mortality, complications, infection, and ICU and hospital length of stay in adult patients with blunt chest trauma.
MethodsThis systematic review was conducted according to the guidelines suggested by the Preference Report Items for Systematic Review and Meta-analyses: the PRISMA statement21 and followed the recommendations of the Cochrane Handbook.22 The study was registered in PROSPERO under the number CRD42022316452.
Data sources and searchesThe search strategy considered the studies published from inception to February 2024 on MEDLINE (PubMed), EMBASE, Cochrane CENTRAL, Web of Science, PEDro and Scielo databases, without restriction of year of publication or language. The following descriptors were used: “Thoracic Injuries”, “Chest Injury” and “Noninvasive Ventilation”, associated with a highly sensitive search strategy for clinical trials.23 Also, there was a manual search in included articles and previous reviews reference lists24,25 and on ClinicalTrials.gov. The full search strategy for each database can be found in Supplementary Material Table 1.
Study selectionTwo reviewers (NCR and RWW) independently evaluated the identified studies and selected them, by title and abstract, according to the following inclusion criteria: 1) randomized clinical trials that evaluated the use of NIV in the treatment of respiratory failure in patients (aged >18 years) with blunt chest trauma, defined as nonpenetrating injury to the chest; 2) at least one comparator group with IMV or oxygen therapy; and 3) studies that evaluated mortality, complications or infections, ICU length of stay, or hospital length of stay as outcome.
Abstracts that indicated that the article potentially met the criteria or that did not provide sufficient information were selected for full-text evaluation. In this phase, the exclusion criteria were: (1) other forms of ventilatory treatment in patients with chest trauma; (2) studies with pediatric patients; (3) published in abstract format only, review articles, case reports; (4) qualitative studies and articles that are not available in full. Disagreements were resolved by consensus and, if necessary, by a third reviewer (AMVS). When only the abstract was available or additional information was needed, emails were sent to the authors once a week for three consecutive weeks.
OutcomesThe outcomes assessed were: mortality, complications (acute respiratory distress syndrome, hemothorax, hemopneumothorax, multiple organ dysfunction syndrome, pneumothorax, rib fracture), infections (pneumonia and sepsis), days of hospitalization in the ICU and hospital, arterial blood gas (partial pressure of oxygen and partial pressure of carbon dioxide) and vital signs (heart rate, respiratory rate, and arterial pressure).
Data extraction and quality assessmentThrough the use of standardized forms, two independent reviewers (NCR and RWW) extracted information on study identification (authors, year of publication, country); sample characteristics (sample size, % of males, age, body mass index, and injury score severity), NIV group data: ventilatory mode, type of interface and time of use; and the comparator group: ventilatory mode and type, flow, and time of use.
Divergences between the evaluators were resolved by consensus, or, whenever necessary, a third reviewer (AMVS) was consulted.
The risk of bias was assessed by two independent reviewers (ALD and NCR), using the tool presented in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0.23(RoB 2.0) for clinical trials.26 The evaluated domains were random sequence generation, concealed allocation, blinding of participants, professionals, and evaluators to outcomes, description of losses or exclusions, and selective reporting. For each of the domains, the risk of bias was characterized as “low”, “high”, or “uncertain”. The certainty in the evidence and strength of the recommendations for each outcome was evaluated according to the grading of recommendation, assessment, development and evaluation (GRADE), considering study design, risk of bias, inconsistency of the results, indirectness, imprecision, and publication bias. The criteria to downgrade the certainty of evidence was: risk of bias - if any study had some concerns or a high risk of bias; inconsistency - when moderate or high statistical heterogeneity was identified; indirectness - when there was a discrepancy between the PICO target elements and the available evidence; imprecision - when the effect size was small or the confidence interval was wide; publication bias - if there was significant bias in the reporting of the studies.27
Data synthesis and analysisRevMan 5.4 software (Review Manager 5.4, The Cochrane Collaboration) was used to perform the analyses. For dichotomous variable (mortality, complication and infection occurrence), odds ratios with a 95 % confidence interval (95 %CI) were calculated by the sample size and the number of events in the NIV group in relation to the comparator group. For continuous variables (days of hospitalization in the ICU and hospital), the mean difference (MD) with 95 %CI was calculated using sample size, mean, and standard deviation. Statistical heterogeneity was quantified using the I2 test, and a value greater than 50 % was considered an indicator of substantial heterogeneity.22 A sensitivity analysis was performed for the outcomes of complications, infections, and ICU and hospital length of stay by excluding a study that used oxygen therapy in the comparator group. One study included two intervention groups (CPAP and BIPAP). In the primary analysis, the BIPAP group was used. A sensitivity analysis was conducted by substituting the CPAP group for the outcomes of ICU and hospital length of stay.
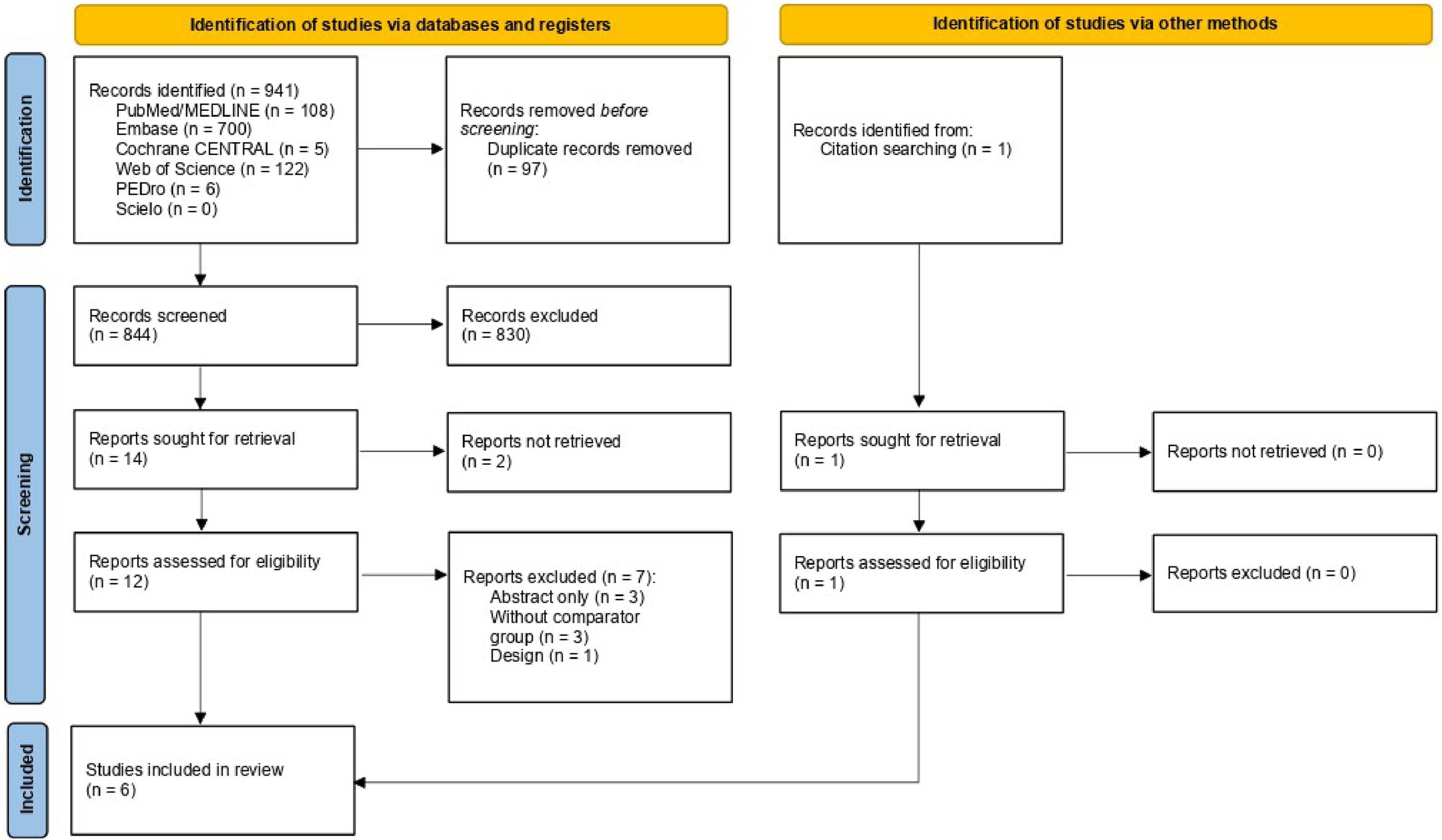
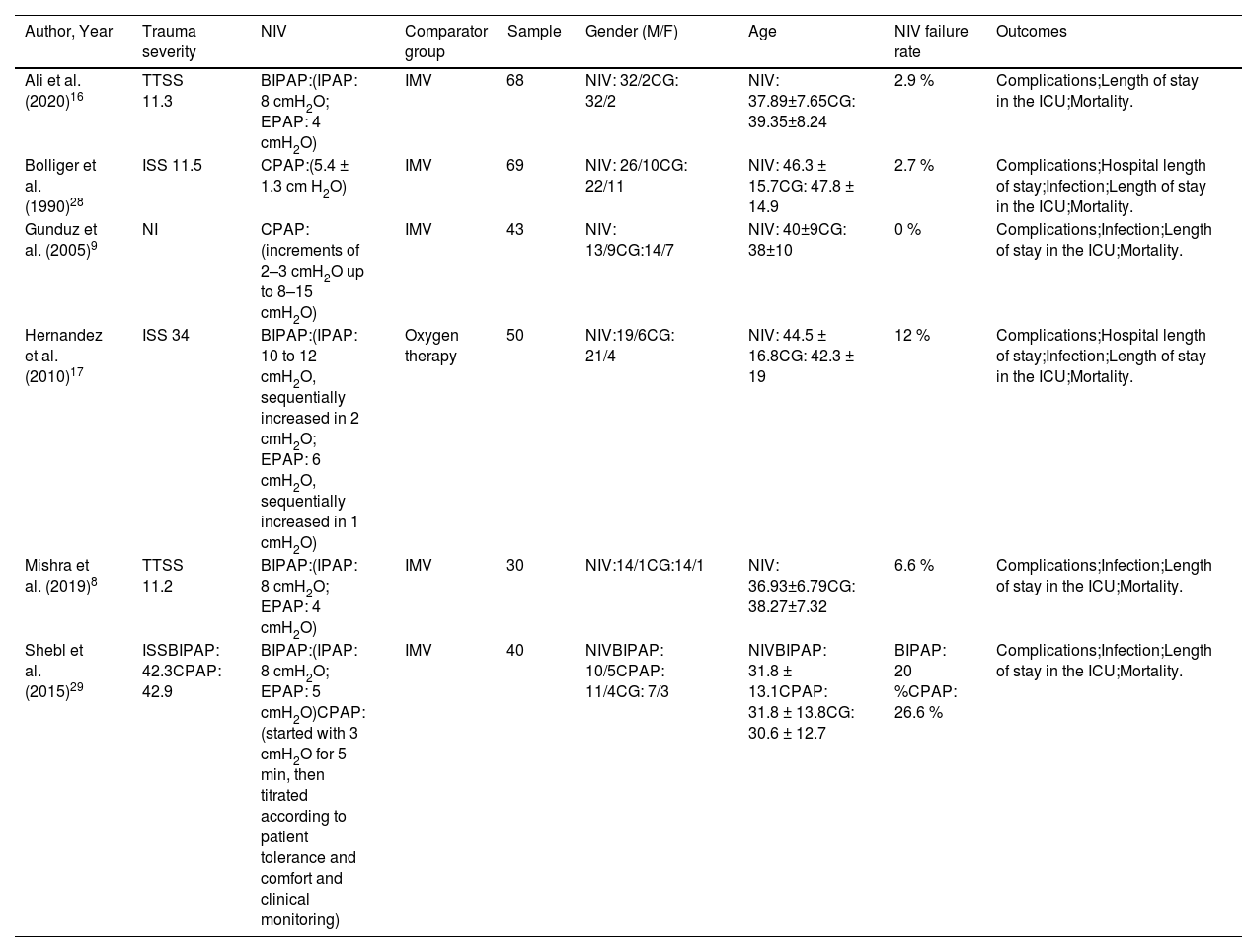
ResultsDescription of studiesInitially, of the 941 potentially relevant studies found, six RCTs8,9,16,17,28,29 met the inclusion criteria. The included studies were published between 1990 and 2020 and included a total of 300 participants (162 in the NIV group, 138 in the comparator groups). Fig. 1 shows the flowchart, detailing the number of studies excluded for each reason and the main characteristics of the studies are detailed in Table 1. For treatment with NIV, three studies used BIPAP,8,16,17 two used CPAP,9,28 and one used BIPAP and CPAP.29 For the comparator treatment, only one study17 used oxygen therapy while the others used IMV.8,9,16,28,29
Characteristics of the included studies.
| Author, Year | Trauma severity | NIV | Comparator group | Sample | Gender (M/F) | Age | NIV failure rate | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Ali et al. (2020)16 | TTSS 11.3 | BIPAP:(IPAP: 8 cmH2O; EPAP: 4 cmH2O) | IMV | 68 | NIV: 32/2CG: 32/2 | NIV: 37.89±7.65CG: 39.35±8.24 | 2.9 % | Complications;Length of stay in the ICU;Mortality. | |
| Bolliger et al. (1990)28 | ISS 11.5 | CPAP:(5.4 ± 1.3 cm H2O) | IMV | 69 | NIV: 26/10CG: 22/11 | NIV: 46.3 ± 15.7CG: 47.8 ± 14.9 | 2.7 % | Complications;Hospital length of stay;Infection;Length of stay in the ICU;Mortality. | |
| Gunduz et al. (2005)9 | NI | CPAP:(increments of 2–3 cmH2O up to 8–15 cmH2O) | IMV | 43 | NIV: 13/9CG:14/7 | NIV: 40±9CG: 38±10 | 0 % | Complications;Infection;Length of stay in the ICU;Mortality. | |
| Hernandez et al. (2010)17 | ISS 34 | BIPAP:(IPAP: 10 to 12 cmH2O, sequentially increased in 2 cmH2O; EPAP: 6 cmH2O, sequentially increased in 1 cmH2O) | Oxygen therapy | 50 | NIV:19/6CG: 21/4 | NIV: 44.5 ± 16.8CG: 42.3 ± 19 | 12 % | Complications;Hospital length of stay;Infection;Length of stay in the ICU;Mortality. | |
| Mishra et al. (2019)8 | TTSS 11.2 | BIPAP:(IPAP: 8 cmH2O; EPAP: 4 cmH2O) | IMV | 30 | NIV:14/1CG:14/1 | NIV: 36.93±6.79CG: 38.27±7.32 | 6.6 % | Complications;Infection;Length of stay in the ICU;Mortality. | |
| Shebl et al. (2015)29 | ISSBIPAP: 42.3CPAP: 42.9 | BIPAP:(IPAP: 8 cmH2O; EPAP: 5 cmH2O)CPAP:(started with 3 cmH2O for 5 min, then titrated according to patient tolerance and comfort and clinical monitoring) | IMV | 40 | NIVBIPAP: 10/5CPAP: 11/4CG: 7/3 | NIVBIPAP: 31.8 ± 13.1CPAP: 31.8 ± 13.8CG: 30.6 ± 12.7 | BIPAP: 20 %CPAP: 26.6 % | Complications;Infection;Length of stay in the ICU;Mortality. | |
BIPAP, Bilevel positive airway pressure; CPAP, Continuous positive airway pressure; IPAP, Inspiratory positive airway pressure; EPAP, Expiratory positive airway pressure; CG, Comparator group; IMV, Invasive mechanical ventilation; ISS, Injury Severity Scale; M/F, Male/female; NI, uninformed; NIV, Noninvasive ventilation - intervention group; TTSS, Thoracic Trauma Severity Score.
The assessment of the risk of bias in the included studies is shown in Supplementary Material Table 2. All studies obtained a classification of some level of concern. The certainty of evidence for each outcome effect estimate was assessed using the GRADE system and ranged from moderate to low certainty (Supplementary Material Table 3).
MortalitySix studies8,9,16,17,28,29 (300 patients) reported information on mortality, of which five studies8,9,16,28,29 (250 patients) compared NIV with IMV. There were 5/137 (3.6 %) deaths in the NIV group compared with 27/113 (23.8 %) in the comparator group (IMV). There was no heterogeneity between studies. The results of the meta-analysis showed moderate certainty (i.e., downgraded due to the serious risk of bias) that NIV likely reduces mortality compared to the comparator group. We found that the odds of mortality among patients treated with NIV is 0.15 times the odds among patients in the comparator group (95 %CI: 0.06, 0.37; p = 0.0001; I2= 0 %; Fig. 2). Only one study17 compared NIV with oxygen therapy and there was no difference in mortality (OR = 1.00; 95 %CI: 0.13, 7.72).
Complications and infectionsSix studies8,9,16,17,28,29 (300 patients) reported complications, of which five8,9,16,28,29 (250 patients) compared NIV with IMV. Five studies8,9,17,28,29 (232 patients) reported infection, of which four8,9,28,29 (182 patients) had IMV as the comparator group. Our meta-analysis showed moderate certainty (i.e., downgraded due to the serious risk of bias), meaning that NIV may reduce complications and infections. The odds of complications among patients treated with NIV is 0.10 times the odds among patients in the IMV group (95 %CI: 0.05, 0.20; p = 0.00001; I2=11 %; Fig. 3A). Similarly, the odds of infections among patients treated with NIV is 0.11 times the odds among patients in the IMV group (95 %CI: 0.05, 0.24; p = 0.00001; I2=0 %; Fig. 3B). Only one study17 compared NIV with oxygen therapy and there were no differences in the rates of complications (OR = 1.64; 95 %CI: 0.53, 5.09) and infections (OR = 1.00; 95 %CI: 0.25, 4.00).
ICU and hospital length of staySix studies8,9,16,17,28,29 (285 patients) reported length of stay in the ICU, of which five8,9,16,28,29 (235 patients) compared NIV with IMV. Our results showed low certainty (i.e., downgraded due to the serious risk of bias and serious inconsistency) that NIV may reduce the length of stay in the ICU. Our results showed that the NIV group stayed 2.29 days less in the ICU (95 % CI −3.78, −0.80, p = 0.003, I2=52 %)] than the comparator group. As the study by Shebl et al29 presents two intervention groups (CPAP and BIPAP), this analysis was carried out using the BIPAP group (Fig. 4). A sensitivity analysis was performed substituting for the CPAP group, showing a similar difference on the length of stay in the ICU [MD −2.46 days (95 % CI −3.81 to −1.10, p < 0.0004, I2=42 %)]. In the only study17 in which NIV was compared to oxygen therapy, there was no difference in length of stay in the ICU (MD = −2.0 days; 95 %CI: −6.27, 2.27).
A meta-analysis of hospital length of stay was not performed because the two studies17,28 reporting this outcome used different comparators (IMV and oxygen therapy). In both studies, there was a reduction in hospital length of stay in the NIV groups compared to the IMV group28 (MD = −6.2 days; 95 %CI: −9.94, −2.46) or with oxygen therapy17 (MD = −7.0 days; 95 %CI: −13.94, −0.06).
Arterial blood gas and vital signsIt was not possible to analyze the variables obtained by arterial blood gas analysis because the studies did not show results before and after the interventions. Of these, three studies8,9,17 analyzed only the pre-installation of NIV or IMV, one study29 only after NIV, and one study28 did not analyze these outcomes. Likewise, vital signs data were not analyzed as only three articles reported some outcomes and did not compare pre and post intervention. These analyzed vital signs only after NIV16,29 or before and after immediate removal of this intervention.8
DiscussionTo our knowledge, this is the first systematic review that used only randomized controlled trials to demonstrate the effects of NIV in reducing mortality, complications, and infections in adults with blunt chest trauma. We also report the effects of NIV in reducing ICU length of stay in this population.
Noninvasive ventilatory assistance provides adequate oxygenation and effective analgesia,11 preventing spontaneous respiratory failure, reducing the need for invasive mechanical ventilation, improving survival in patients with chest trauma,30 pulmonary recruitment and reducing intubation and mortality rates.18,31 Corroborating these findings, several authors8,9,16,29 found decreased mortality in patients with chest trauma who used NIV compared to IMV.
Chest trauma is continually increasing and the cause of mortality and morbidity in blunt chest trauma is mainly due to pulmonary complications.32 Previous review demonstrated that patients receiving NIV had a non-significant reduction in the risk of death. However, only three studies were included.19 In this systematic review, we highlight the use of NIV as a major strategy to reduce mortality in the group of patients who suffered chest trauma, with only 4.3 % of deaths occurring in the group that used NIV, compared to 21 % of deaths in the group that received IMV or oxygen therapy.
Airway obstruction, tension pneumothorax, open pneumothorax, massive hemothorax, flail chest, and cardiac tamponade are the six deadly conditions that can be seen in chest trauma.33 Management in patients with blunt chest trauma is primarily provided by early mobilization, adequate pain control, adequate fluid resuscitation, and adequate respiratory support.33 The first modern approach for flail chest was established by Avery et al. ,34 who reported that continued mechanical ventilation is required for internal stabilization of chest wall.
Our meta-analysis showed the effectiveness of NIV in reducing complications and infections after chest trauma, which may be attributed to the lower risk of lung collapse and early-onset pneumonia. With exception, in the study by Hernandez et al17 pneumothorax tended to be greater in the NIV group, although this difference did not reach statistical significance. The higher rate of pneumothorax in patients with NIV probably results from using slightly higher airway pressures for longer periods.17 NIV can significantly reduce the risk of developing acute respiratory distress syndrome due to its role in lung recruitment and/or prevention of lung infections.35
In 1982, Linton e Potgieter36 already found that conservative management of blunt chest trauma reduces complications and this should be attributed to the correction of the respiratory defect through conservative management and prevention of tracheal invasion and invasive mechanical ventilation. The importance of this treatment is also determined by fewer side effects compared to invasive ventilation, which is associated with higher rates of nosocomial pneumonia and prolonged mechanical ventilation.4,18
These findings are in line with several studies8,9,16,28 which found a decrease in complications in the group of patients who used NIV. The main complications found were pneumothorax and acute respiratory distress syndrome. A previous study has already reported that NIV is preferred over invasive ventilation because it helps to avoid many of the complications associated with intubation and tracheostomy.37 Three studies8,9,28 included in our meta-analysis also reported a lower chance of pneumonia and sepsis with the use of NIV. Thus, our findings demonstrated that NIV reduces complications and infections associated with chest trauma, which can result in a drop in hospital costs and impact savings on health services.
The reduced ICU and hospital length of stay in patients with chest trauma using NIV is expected, as intubation increases the risk of infections, complications, and prolonged ICU stay.38 Likewise, Ferrer et al30 found that hospital stay among ICU survivors decreased in the NIV group. Other authors36 attribute differences in length of stay and morbidity between NIV and IMV groups to the correction of the respiratory defect through conservative management and prevention of tracheal invasion and mechanical ventilation.
Another major justification for the difference in ICU length of stay is attributed to sedation, in which invasively ventilated patients receive continuous sedation, while the non-invasive group receives only epidural anesthesia.9,28,36 As these studies did not use spontaneous breathing tests and sedation interruption, the duration of endotracheal intubation was probably an important factor in the length of stay in the ICU.39
In the systematic review by Chiumello et al.,18 the length of stay in the ICU was also significantly shorter in the group of patients who used NIV, while the length of hospital stay was shorter, but without reaching significance. In the review conducted by Roberts et al., patients who received NIV demonstrated a significant reduction in ICU and hospital length of stay.19 In this systematic review, the length of ICU stay was shorter in the NIV group compared to the IMV group. It was not possible to analyze hospital length of stay, but in the two included trials, NIV was better than IMV or oxygen therapy. In contrast to these findings, Udekwu et al40 showed that patients undergoing NIV had longer ICU and hospital stays, but there was no increase in mortality or respiratory failure. They attributed the longer length of stay in patients with NIV to treatment bias. Although they were able to statistically control for rib fracture pattern and age, they did not include an objective measure of physical frailty. They hypothesized that frailty was a determining factor in the decision to place patients on NIV, and that this unmeasured variable could be responsible for differences between groups in length of stay.
The reduction in ICU stay in the groups treated with NIV, evidenced by our findings, stands out as a relevant and patient-centered outcome. Early discharge tends to mitigate the risks of infections and complications, hospitalization costs, and the burden on health services.41 Decisions regarding the choice of ventilatory support should be individualized and made in collaboration with the healthcare team.42 In the absence of contraindications, NIV can be used as a first choice ventilatory support.43 However, in cases of NIV failure, confirmed by continued impairment of gas exchange or deterioration of respiratory function, invasive mechanical ventilation is required.42,43
Among the limitations of the studies we can consider the small sample, few clinical trials, and study heterogeneity. It was not possible to analyze any results regarding gas exchange or vital signs, even though these outcomes were relevant to establishing or maintaining the patient using NIV. Given the risk of bias analysis and the low to moderate quality of evidence for the analyzed outcomes, new clinical trials are necessary.
Despite this, our study has important clinical implications, as NIV is a lower-cost ventilatory assistance compared to other healthcare procedures. In this way, its use in the emergency care units or ICU can improve therapeutic management and mitigate multisystem complications that can result from the worsening of conditions secondary to thoracic trauma. Furthermore, the combination of favorable and patient-centered results is highly encouraging for the use of NIV, enabling earlier hospital discharge and, possibly, better functional conditions after blunt chest trauma, thereby contributing to physical therapists' clinical practice by offering evidence-based guidance for respiratory care and rehabilitation strategies.
ConclusionsThis systematic review with meta-analysis using only randomized clinical trials validates the use of NIV in blunt chest trauma. In clinical practice, NIV can promote a reduction in mortality, complications, infection, and length of stay in the ICU when compared to the use of IMV.
FundingThis study was partially funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (Finance Code 001). The funder played no role in the design, conduct, or reporting of this study.
The authors declare no competing interest.