Acute pain has been tremendously beneficial throughout evolution as it enables us to identify potential harmful stimuli, and as it ensures we protect damaged tissue while it heals. However, once evolved into a chronic state, its protective role diminishes with a wide range of negative and maladaptive sequelae that massively impact both the individual and society. Neuroscientific research has significantly advanced our understanding about pain and chronic pain in particular, including the role of central (nervous system) sensitization in the generation and amplification of (persistent) pain experiences. This knowledge innovation created a massive implementation potential but also a challenge for clinicians to remain up-to-date in daily practice. Particularly the variety of and rapid change in concepts and terminology used can be challenging for clinicians. For these reasons, this second part of the comprehensive pain management editorial series1 provides a terminology update regarding central sensitization and nociplastic pain in a clinically applicable way. Essentially, central sensitization is the major underlying mechanism of nociplastic pain, which is a pain phenotype.
Human assumed central sensitization: definition and assessmentMany patients with chronic pain lack a clear origin of nociceptive input, or the input demonstrated does not suffice to explain the experienced pain, disability and other symptoms. In such patients, central sensitization can explain the chronic pain experience. Central sensitization is defined as “an amplification of neural signaling within the central nervous system that elicits pain hypersensitivity”.2 To prevent clinicians becoming lost in translation, clinicians and clinical researchers are advised to adhere to this definition as it allows studying central sensitization in humans; while other definitions such as “an increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input”3 are incompatible with the intentions of the initial preclinical central sensitization discovery studies to improve understanding of chronic pain in humans.2 Indeed, in vivo measurements of nociceptive neuron responses are not possible, and would imply that central sensitization stays in the (animal) lab, merely limiting its implementation in humans to hypothesis-based applications (Table 1). Luckily, clinicians and clinical researchers around the globe agreed that central sensitization should not stay in the lab. This resulted in hundreds of studies examining features of human assumed central sensitization in a variety of chronic pain conditions, which in turn lead to central sensitization now being a well-established feature in many patients with chronic pain including those with low back pain, neck pain, headache, post-cancer pain, fibromyalgia, osteoarthritis, pelvic pain, post-surgical pain, and pediatric pain (reviewed in Nijs et al. 20214). In those chronic pain conditions, central sensitization can be a feature of the condition or presented clinically in a subgroup of patients.
Misconceptions about central sensitization and nociplastic pain.
| Misconception | Correct statement facilitating uptake by clinicians |
|---|---|
| Central sensitization cannot be assumed in humans. | (Clinical) features of central sensitization (e.g., spreading of pain, pain hypersensitivity, sensitivity to touch, movement, pressure, or heat/cold) can be assessed in humans and can be assessed by clinicians. |
| Central sensitization can only be assessed in preclinical (animal) studies. | Features of central sensitization have been identified in hundreds of studies in a variety of chronic pain conditions.4 |
| Central sensitization should stay in the lab. | Central sensitization was never intended to stay in the lab; it was always meant to serve health care for patients with chronic pain by improving understanding of its underlying mechanisms. |
| Central sensitization and nociplastic pain are synonyms. | Central sensitization is one of the major underlying mechanisms of nociplastic pain, but they are not synonyms. Nociplastic pain is a pain phenotype associated with many features of central sensitization. |
| Central sensitization is only seen in patients with nociplastic pain. | Central sensitization is also a key underlying mechanism of neuropathic pain, and features can be found in subacute pain as well. |
| Central sensitization entails ‘psychological pain’. | Cognitive-emotional factors play a major role in any pain experience, and contribute to the sensitized central nervous system seen in many patients with chronic pain. |
Central sensitisation should be viewed as an umbrella term covering several, partly overlapping and highly related mechanisms. Indeed, central sensitization encompasses various related dysfunctions within the central and peripheral nervous system, including altered sensory processing in the brain5 with the brain showing increased activity in the network identifying stimuli that deserve our attention.6 Such altered brain activity pattern is thought to be responsible for orchestrated nociceptive facilitatory pathways5,7 and poor functioning of endogenous analgesia,8,9 two other features of central sensitization. Together, these central nervous system dysfunctions not only contribute to increased responsiveness to a variety of sensory inputs, such as tactile stimuli, but can also lead to hypersensitivity to non-musculoskeletal stimuli, such as chemical substances, odours, light, sound, heat, cold, touch, stress, and electricity.10 Indeed, a key message for clinicians is that central sensitisation goes beyond the nociceptive system and pain experience.11
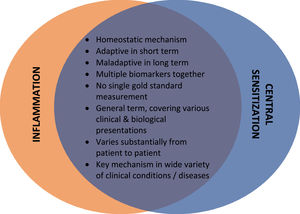
To facilitate the understanding of the concept of central sensitization, it is crucial to understand that central sensitization is a homeostatic mechanism. This implies, similar to inflammation, that central sensitization is adaptive in the short term (e.g., in the days following surgery or following a motor vehicle accident). As such, it avoids further deterioration in acute pain situations. However, central sensitization becomes maladaptive in the long term, with pain losing its protective value as commonly seen in patients with persistent pain (e.g., months after successful surgery or a motor vehicle accident). In addition, central sensitization and inflammation – although physiologically very divert mechanisms – share many features, including the notion that multiple biomarkers together rather than a single gold standard are required for assuming its presence, the fact that they are both umbrella terms covering various clinical & biological presentations, that their clinical presentation varies substantially from patient to patient, and that they are both key mechanism in a wide variety of clinical conditions/diseases (Fig. 1).
Central sensitization: a paradigm shift in understanding & managing chronic painThe knowledge regarding central sensitization has revealed a paradigm shift in the understanding and management of chronic pain that allows clinicians to think beyond muscles and joints,12 and to account for the role of pain modulation in the central nervous system.4 Importantly, central sensitization provides a common mechanism potentially explaining complex persistent pain conditions and its various clinical presentations, including common comorbid symptoms. This has been implemented globally through provision of pain neuroscience education, which often includes (but is not limited to) explaining central sensitization as the underlying mechanism for the patient's pain condition. In addition, the paradigm shift in the management of chronic pain also includes a shift away from tissue-targeted interventions, and a renewed and strengthened focus on management strategies and interventions that aim for a healthier lifestyle and normalization of central nervous system functioning. When providing such ‘updated’ interventions, it is cardinal to take the mechanism of central sensitization into account, rather than aiming for ‘treating’ it. This implies targeting perpetuating factors such as pain catastrophizing, illness perceptions, fear, anxiety, depression, maladaptive coping strategies, perceived injustice, and lifestyle factors including poor sleep, stress, diet, smoking, and physical inactivity. Taking central sensitization into account when treating patients with chronic pain also implies not relying on short-term changes in pain (severity) in response to interventions. Short-term changes in pain severity should not be relied upon when measuring the success of therapy such as exercise therapy, nutritional interventions, or any aspect of psychologically-informed practice (e.g., stress reduction interventions, cognitive behavioural interventions). Recognizing central sensitization as a key mechanism explaining the patient's pain and suffering implies that pain is no longer a reliable messenger. Moreover, adapting (exercise or other) interventions to variances in pain will reward the brain in producing pain. Hence, exercise therapy and physical activity using a time-contingent approach is warranted in these patients.13 Cumulating evidence suggests that physical therapy (e.g., exercise therapy, manual therapy) produces slight improvements in features of central sensitization in patients with chronic musculoskeletal pain.14
Nociplastic painThe presence of clinical features of central sensitization predicts poor treatment outcomes in patients with a variety of chronic pain conditions,15-19 at least when the treatment targets local tissues/ the presumed source of nociception. This applies to conservative interventions,18,19 but also to surgical interventions.20-23 These observations illustrate the need for early recognition of central sensitization in patients with chronic pain, in combination with tailored treatment.24 This brings us to the term “nociplastic pain”, which was introduced in 2017 as a third mechanistic pain descriptor (i.e., pain phenotype) in addition to nociceptive and neuropathic pain.25,26 It is important to stress the differences between nociplastic pain and central sensitization. While central sensitization is the major underlying mechanism of nociplastic pain,25,27 it is also common in neuropathic pain.28 Nociplastic pain is defined by the International Association for the Study of Pain (IASP) as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain”.26 The relevance of the term lies in the recognition that not all chronic pain conditions can be explained solely by structural or tissue damage. By acknowledging the existence of nociplastic pain, clinicians and researchers aim to improve understanding, diagnosis, and management of these conditions. Nociplastic conditions can be challenging to diagnose and treat because they lack clear objective markers and are often associated with complex underlying mechanisms. To account for this challenge, in 2021 the IASP released the first set of clinical criteria and a grading system for nociplastic pain.27 These criteria allow clinicians to identify and correctly classify patients with chronic pain according to their pain phenotype, in order to meet the patients’ need for appropriate pain treatment and improve precision pain medicine practices. The clinically highly relevant and timely issue of phenotyping patients into nociceptive, neuropathic, and nociplastic pain will be addressed in more detail in the next edition of the Comprehensive Pain Management Editorial Series.