Monochromatic infrared energy (MIRE) or phototherapy has been used to improve plantar sensitivity and pain in lower limbs of patients with diabetic sensorimotor peripheral neuropathy (DSPN), but the available primary results are inconsistent.
ObjectiveTo review systematically the effects of MIRE on plantar sensitivity and neuropathic pain in patients with DSPN.
MethodsMedline, EMBASE, Cochrane CENTRAL, and Google Scholar were searched up to September 2016. Randomized controlled trials addressing the effects of MIRE on plantar sensitivity and neuropathic pain in patients with DSPN were selected. Study inclusion, risk of bias and quality assessment, and data extraction were completed by two independent reviewers.
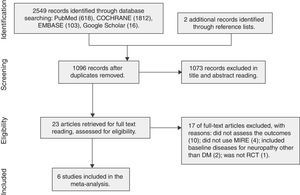
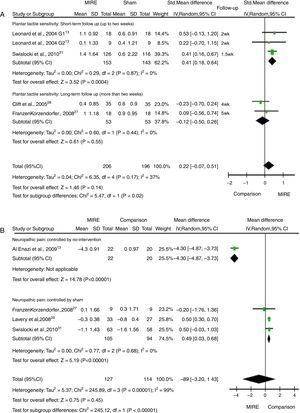
ResultsOf 2549 records identified, six studies met the selection criteria, with 304 patients (594 feet) randomized. MIRE was not associated with improvement in plantar tactile sensitivity (SMD=0.22, 95%CI −0.07 to 0.51, low quality of evidence). Subgroups of studies with short-term (up to 2 weeks) follow-up showed significant improvement in plantar sensitivity (SMD=0.41, 95% CI 0.18–0.64). Neuropathic pain increased significantly in patients who received MIRE (MD=0.49, 95% CI 0.30–0.68, low quality of evidence).
ConclusionsThere was limited evidence that MIRE results in short-term improvement of tactile sensitivity probably not sustained over time. Limited evidence also suggested that MIRE does not provide relief for neuropathic pain. As quality of evidence is low, further studies are likely to change the estimated effect.
Diabetic chronic sensorimotor distal symmetrical polyneuropathy (DSPN) is considered one of the most common long-term microvascular complications of diabetes mellitus regardless of type,1 affecting up to 50% of people with diabetes.2,3 The onset of DSPN is usually insidious and heralded by sensory symptoms such as numbness and diminished sensation in lower limbs that start in the toes and progress proximally in a symmetrical distribution.2,3 Symptoms are present in 50% of patients with DSPN, with 10–26% presenting with neuropathic pain.3 Decreased feet sensitivity associated with DPSN is a significant risk factor for subsequent diabetic ulcers and non-traumatic amputations.4 These complications lead to impairments in quality of life and high consumption of healthcare resources.5
The most effective approach to preventing DSPN and its complications consists in strict control of diabetes.6,7 Once developed, few specific interventions are available for treatment8,9 and the management of neuropathic pain is limited due to adverse effects.3,8,9 Consequently, new adjunctive strategies have been proposed. Monochromatic Infrared Energy (MIRE), delivered through light-emitting diodes (LED), is one of these complimentary therapies. MIRE is approved by the Food and Drug Administration as a complimentary strategy to improve blood perfusion and reduce pain,10 and it is used in patients with DSPN to improve plantar sensitivity and pain symptoms.11–13
The mechanisms by which MIRE produces its biological effects remain unclear. As infrared wavelengths penetrate to a greater depth than visible light,14 it is suggested that photostimulation of hemoglobin increases nitric oxide release, leading to blood flow improvement.15–18 Other biological effects of MIRE may be the enhancement of cell metabolism, by stimulating mitochondrial ATP production, accelerating antioxidant mechanisms, and improving cell function.19–22
Uncontrolled11,12,23–26 and controlled13,27–32 clinical trials assessed the effects of MIRE on plantar sensitivity restoration and pain relief in patients with DSPN, but results are inconsistent and the efficacy of this intervention has not yet been established for this population. Despite controversies about its effects, the use of LED technology is increasing, mostly in home settings, boosted by its low cost and easy access. Therefore, we conducted this systematic review in order to evaluate better the current evidence about the effect of MIRE on plantar sensitivity and neuropathic pain in patients with DSPN.
MethodsProtocol and registrationWe performed this systematic review in accordance with the Cochrane Handbook for Systematic Reviews of Interventions33 and followed the tutorial of the Brazilian Journal of Physical Therapy.34 The protocol of this systematic review was registered at PROSPERO (CRD42013005068), and it can be assessed online.35
Eligibility criteriaWe included randomized controlled trials (RCT) evaluating the effect of MIRE on plantar tactile sensitivity and/or neuropathic pain in the lower limbs in patients with DSPN. Trials should have at least one intervention group and one comparison group. MIRE was defined as if delivered through LED with wavelengths in the infrared or near-infrared range (750–1300nm).20 The comparison group should not have been exposed to MIRE. Our primary outcome was plantar tactile sensitivity assessed by Semmes–Weinstein monofilament (SWM). Secondary outcome was neuropathic pain in the lower limbs. The exclusion criteria were intervention performed in other regions than feet and follow-up intervention fewer than three days.
Search strategiesWe searched in the electronic databases MEDLINE (accessed by PubMed), Cochrane CENTRAL, and Google Scholar and in EMBASE (up to September 30, 2016). The search terms included MeSH and entry terms related to diabetic neuropathy, infrared-ray, and a methodological filter for RCTs.36 Outcome terms were not included to enhance the search sensitivity. There were no restrictions regarding language or publication date. The terms were adjusted to fit the requirements of each electronic database. We screened the list of references of included studies in order to identify additional RCTs. The complete search strategy applied on PubMed database can be assessed online in the systematic review protocol.35
Study selection and data extractionTwo reviewers (CCR, PSK) separately and independently screened the titles and abstracts of studies identified in the initial search. A standard screening checklist based on the eligibility criteria was employed for each study. Studies not meeting eligibility criteria according to titles or abstracts were excluded. Full texts of the remaining studies were retrieved for a second review independently by the two reviewers. For studies without sufficient information to evaluate the eligibility criteria, we contacted the authors by email to obtain additional information.
We extracted the following data from the included studies: description of study participants, intervention in the experimental group and in the control group, and description and results of the outcomes measurements. Two review authors (CCR, PSK) independently extracted the data from the eligible studies. Discrepancies were solved through discussion and a third author (CS) was consulted when consensus was not reached. We contacted the authors by email to obtain additional information when studies presented incomplete descriptions. Procedures for estimation of missing data33 were performed when possible. If data was still insufficient after these processes, the outcome was included in descriptive analysis only.
Quality of evidenceTwo review authors (CCR, PSK) independently assessed the risk of bias of the included studies using the Cochrane Risk of Bias Tool.33 The following items were evaluated for each study: selection bias (random sequence generation and allocation concealment), performance bias (blinding of patients and investigators), detection bias (blinding of outcome assessors), attrition bias (description of losses and exclusions), and reporting bias (selective reporting). We used the grading of recommendation, assessment, development, and evaluation (GRADE) system to evaluate the quality for the body of evidence.37
Data analysisIn order to standardize units of measurement, the average insensitive plantar regions to the 5.07 SWM were converted to average sensitive plantar regions. Since the two groups in the study of Leonard et al.13 were composed of different individuals, they were included independently in the meta-analysis. Data imputed in the analysis of Swislocki et al.31 was the average effect between feet as originally the study assessed plantar sensitivity for the left and the right foot separately. Pain scale of one study29 was converted from the range 0–100 to 0–10.
For quantitative synthesis, results were pooled using mean difference (MD) when appropriate. As plantar tactile sensitivity measurement was inconsistent across trials, data was pooled using standardized mean difference (SMD). The change value, post minus pre intervention, was used for all the analysis. For meta-analysis, we pooled random-effect model, with DerSimonian and Laird as variance estimator.
Statistical heterogeneity of the effects across studies was assessed using Cochran's Q test and Inconsistency I2 test. Values above 25% and 50% were considered as indicatives of moderate and high heterogeneity. To explore the statistical heterogeneity, subgroup analyses were carried out considering the type of comparison (no-intervention or sham) and length of follow-up intervention: short-term follow-up (up to two weeks) or long-term follow-up (more than two weeks). A p value ≤0.05 was considered statistically significant. All analyses were conducted using Review Manager, version 5.3.
ResultsDescription of studiesThe search strategy yielded 2549 registers, 23 of which were considered as potentially relevant and were retrieved for detailed analysis. Fig. 1 shows the inclusion processes. Six studies, with a total of 304 patients with DSPN, met the eligibility criteria for the systematic review. Some studies randomized feet for plantar sensitivity outcome (total of 594 feet). Table 1 summarizes the characteristics of these studies.
Characteristics of the included studies.
| Author, Year | Intervention device | Participants | Comparator | N IG/CG | Age (sd) IG/CG | Gender masc. IG/CG | Protocol | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Leonard et al.,13 2004 | ATS-480 (Anodyne Therapy, Tampa, USA). Four pads with 60 near-infrared gallium aluminum arsenide diodes array (890nm; 1.3J/cm2/min). | Type 1 and 2 DM – G1: able to sense the SWM 6.65 in five plantar areas; G2: unable to sense the SWM at no less than one plantar area. | Contralateral limb sham (device with inactive diodes) | G1: 18/18; G2: 9/9 Feet | G1: 61 (12); G2: 64 (9) | G1: 12; G2: 7 | A total of 6 sessions in 2 weeks (one limb with active diodes and one limb sham): 3 days/week, 40min/day. Plus 6 sessions in 2 weeks (both limbs with active diodes). Pads placement: on the top and on the bottom of the foot and on each side of the calf just above the ankle. | Plantar tactile sensitivity (average sites insensate to the 5.07 SWM per foot – 0 to 5). Neuropathic pain: overall lower limbs pain level at 10-point VAS. |
| Cliff et al.,28 2005 | ATS-120 (Anodyne Therapy, Tampa, USA). Four pads with 60 near-infrared gallium aluminum arsenide diodes array (890nm; 1.95J/cm2/min). | DM | Sham group (device with inactive diodes) | 18/18 Patients 35/35 Feet | 63.9 (9.6)/63.6 (10.2) | 25/34 | A total of 12 sessions in 4 weeks: 3 days/week, 30min/day. Follow up after 4 weeks. Pads placement: distal posterior leg, distal anterior leg, plantar foot over metatarsals heads, and plantar arch foot. | Plantar tactile sensitivity (average sites sensate to the 5.07 SWM per foot – 0 to 4). Neuropathic pain: not assessed. |
| Franzen-Korzendorfer et al.,27 2008 | ATS-120 (Anodyne Therapy, Tampa, USA). Four pads of infrared diodes array (1.5J/cm2/min). | DM | Contralateral limb sham (device with inactive diodes) | 18/18 Feet | 65 (13) | 12 | A total of 12 sessions in 3 to 5 weeks: 2–5 days/week, 30min/day. Pads placement: dorsal and plantar surfaces of the foot, medial and lateral sides of each lower leg. | Plantar tactile sensitivity (average sites sensate to the 5.07 SWM per foot – 0 to 5). Neuropathic pain: pain level at 10-point VAS per lower limbs. |
| Lavery et al.,29 2008 | ATS-480 (Anodyne Therapy, Tampa, USA). Four pads with 60 near-infrared gallium aluminum arsenide diodes array (890nm; 1.3J/cm2/min). | DM | Sham group (device delivering heat at 37°C and with inactive diodes) | 33/27 Patients | 65.7 (1.9)/64.2 (2.0) | 35%/0.2% | A total of 90 sessions in 12 weeks: 7 days/week, 40min/day. Pads placement: two pads in the plantar aspect of the foot in a T formation on the lateral and medial side of the calf. | Plantar tactile sensitivity (level of sensation by using SWM per foot – 0 to 10 plantar areas). Neuropathic pain: pain level at 100-point VAS per lower limbs. |
| Al Enazi,30 2009 | ATS-480 (Anodyne Therapy, Tampa, USA). Four pads with 60 near-infrared gallium aluminum arsenide diodes array (890nm; 1.3J/cm2/min). | Type 2 DM | Control group – without any active intervention | 22/20 Patients | 58.1 (4.7)/60.4 (9.0) | 12/8 | A total of 12 sessions in 4 weeks: 3 times/week, 30min/day. Pads placement: distal posterior and anterior leg, plantar foot over metatarsal heads, and plantar arch of foot. | Plantar tactile sensitivity (level of sensation by using 5.07 SWM per foot – unclear procedure). Neuropathic pain: pain level at 10-point VAS per lower limbs. |
| Swislocki et al.,31 2010 | C3 DPN BiPhase Photo Stimulator (C3 Medical Technologies, Lafayette, USA). Sixteen pads with of 32 LEDs array (870nm; 1800J/7min) | Type 1 and 2 DM | Sham group (device with inactive diodes) | 63/58 Patients | 64.1 (10.5)/64.4 (8.7) | 84.5%/76.2% | A total of 4 sessions in 1.5 week: 7min/day. Pads placement: dorsum of the foot, between all the toes, anterior and superior to the medial side of the calcaneum tuberosity, above the medial malleolus, posterior to the border of the tibia; between the tip of the malleolus and the Achilles tendon, at the midpoint of the transverse popliteal crease, between the tendons of the biceps femoris and semitendinosus; nine pairs on the plantar surfaces. | Plantar tactile sensitivity (average sites sensate to the 5.07 SWM per foot – 0 to 10). Neuropathic pain: pain level at 10-point VAS per lower limbs. |
IG, intervention group; CG, control group; DM, diabetes mellitus; G1, group 1 (sensate); G2, group 2 (insensate); SWM, Semmes-Weinstein monofilament; LED, light-emitting diodes; VAS, visual analog scale.
Five studies13,27–29,31 compared MIRE to sham intervention, and one study30 compared MIRE to no intervention. Regarding the sham comparisons, two studies13,27 considered the foot as the unit of randomization, and three studies28,30,31 randomized the patients. MIRE wavelengths ranged from 870 to 890nm with an energy density ranging from 1.30 to 1.95Joulescm2min−1. Follow-up length ranged from 1.531 to 12 weeks29; median follow-up was four weeks. Session duration ranged from seven to 40min/day. Interventions occurred in outpatient environment.
Quality of evidenceAll of the included studies showed adequate sequence generation, but none reported information about the allocation concealment. Out of six studies, one30 did not blind patients and investigators and 50% reported blinding of outcome assessors. Selective reporting was identified in one study that performed analysis per protocol,29 however 60% of studies did not report the type of analysis. Description of losses and exclusions were reported in 50% of the studies (Table 2). Quality of evidence, rated using GRADE, ended with low-quality evidence for both the outcomes. The evidence profile is presented in Table 3.
Risk of bias of the included studies.
| Author, Year | Adequate sequence generation | Allocation concealment | Blinding of patients and investigators | Blinding of outcome assessors | Selective reporting | Description of losses and exclusions |
|---|---|---|---|---|---|---|
| Leonard et al.,13 2004 | Low | Unclear | Low | Unclear | Unclear | Unclear |
| Clift et al.,28 2005 | Low | Unclear | Low | Low | Unclear | Low |
| Franzen-Korzendorfer et al.,27 2008 | Low | Unclear | Low | Low | Unclear | Unclear |
| Lavery et al.,29 2008 | Low | Unclear | Low | Unclear | High | High |
| Al Enazi,30 2009 | Low | Unclear | High | High | Unclear | Unclear |
| Swislocki et al.,31 2010 | Low | Unclear | Low | Low | Low | Low |
Quality of the evidence.
| Quality assessment | No. of patients | Effect | Quality | Comment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | MIRE | Control | |||
| Plantar sensitivity (follow-up range: 1.5 weeks to 12 weeks; assessed with: number of plantar regions sensitive to 5.07 SWM) | ||||||||||
| 6 | RCT | Not seriousa | Seriousb,c | Seriousd,e | Not serious | 206 | 196 | SMD 0.22 SD higher (0.07 lower to 0.51 higher) | ⊕⊕○○ LOW | Data was pooled for four studies. Two studies29,30 assessed plantar sensitivity by level of sensitivity and analysis was descriptive. |
| Neuropathic pain (follow-up range: 1.5 weeks to 12 weeks; assessed with: VAS scale) | ||||||||||
| 3 | RCT | Not seriousa | Not serious | Seriousf,g | Serioush | 127 | 114 | MD 0.49 higher (0.3 higher to 0.68 higher) | ⊕⊕○○ LOW | An additional trial evaluated the outcome of interest30 was excluded from the analysis because of lack of blinding. |
RCT, randomized controlled trial; CI, confidence interval; SMD, standardized mean difference; MD, mean difference.
Quality of evidence was not downgraded for risk of bias since most of the studies were blinded and no major problems with randomization were detected; concerns about length of follow-up and method of measurement were considered in other domains.
Measurement of plantar sensitivity was not performed using a gold standard method; monofilament was used instead.
Six studies evaluated plantar tactile sensitivity. Of those, four studies13,28,30,31 assessed plantar sensitivity by measuring the number of plantar regions sensitive to the 5.07 SWM. Pooled-effect for these four studies included a total of 402 feet. MIRE did not increase the number of plantar regions sensitive to the 5.07 SWM compared to the control group [SMD=0.22 (95% CI: −0.07 to 0.51, I2: 37%), low quality of evidence] (Fig. 2A). Subgroup analysis showed the benefit of MIRE in studies with follow-up of two weeks or less [SMD=0.41 (95% CI: 0.18–0.64, I2: 0%)] (Fig. 2A). This effect was not sustained after the two weeks [SMD=−0.12 (95% CI: −0.50 to 0.26, I2: 0%)] (Fig. 2). The difference for these subgroups was statistically significant (p=0.02) (Fig. 2A).
Two studies29,30 assessed plantar tactile sensitivity by measuring the level of sensitivity. Lavery et al.29 used different sizes of SWM (4.56, 5.07, 5.46, and 5.88) to evaluate the level of sensation at 10 regions on each foot. After the 12-week follow-up intervention, MIRE had no effect in improving the overall level of perception compared with sham. Conversely, when compared to a non-intervention control group, Al Enazi30 reported that MIRE significantly improved plantar sensitivity level after four weeks of follow-up [SMD: 1.93 (95% CI: 2.68–1.19]. This study assessed level of plantar sensitivity with 5.07 SWM but the description of outcome measurement was unclear.
Neuropathic painFour studies,27,29–31 with a total of 241 patients, were included in the meta-analysis for neuropathic pain. Studies assessed neuropathic pain intensity in lower limbs using a visual analog scale (0–10 or 0–100). Overall pain symptoms did not differ between MIRE and comparison groups, however high heterogeneity was observed [MD: 0.80 (95% CI: -3.20 to 1.43, I2: 99%)] (Fig. 2B). After excluding a non-blinded study controlled by no intervention, MIRE resulted in a lower decrease in pain intensity compared to sham [MD: 0.40 (95% CI: 0.30–0.68, I2: 0%), low quality of evidence] (Fig. 2B).
DiscussionSummary of the evidenceThere was limited evidence that MIRE increased plantar tactile sensitivity on the first two weeks of intervention and that such effect was not sustained over time. This short-term effect is expected, given the natural history of DSPN featured by elevated thresholds of vibration and thermal perception that evolves to tactile sensory loss due to degeneration of all types of peripheral nerves.38 In addition, initial improvement of nerve fiber function by MIRE might have been responsible for enhancing the sensitivity of sensory pain nerve fibers, which in turn can be related to the lower decrease in pain relief in patients receiving MIRE compared to those received sham intervention. Management of pain is challenging in DSPN as decreasing of symptoms is associated with receptor degeneration and worsening of sensory function.39
Furthermore, a placebo effect cannot be ruled out. MIRE resulted in larger effects on pain relief and plantar tactile sensitivity in Al Enalzi,30 the only non-blinded study controlled by a non-intervention group, in comparison with all of the other sham-controlled blinded studies. It is important to clarify that sham intervention would avoid the placebo effect of MIRE and adequate blinding would prevent measurement bias, critical for subjective outcomes such as pain and sensitivity.40 There are no significant differences in patient characteristics or in MIRE treatment that could explain the larger effect found in Al Enalzi30 other than the non-blinded, non-intervention comparison. Thus, we assumed that this was the main bias related to the effect size found in this study.
Plantar sensitivity assessment by SWM is a good screening test for DSPN and it is useful for risk prediction for foot ulceration.38 On the other hand, this measure might not be quite as accurate to detect changes in sensory nerve fiber function over time. Nevertheless, the identified studies used this measure as a primary endpoint for assessment of sensitivity restoration. Accurate measures for nerve function, such as nerve conduction velocity and quantitative sensory testing, should be used as primary outcomes measurement in further studies.
The visual analog scale is one of the oldest, easiest, and best validated tools to assess pain but evaluation of neuropathic pain requires the understanding of the possible negative (sensory loss) and positive (pain and paresthesia) symptoms and signs.41 Only Swislocki et al.31 assessed quality of pain (by using the Neuropathic Pain Scale) and interestingly showed a decrease in tingling, cramping, and itchy symptoms in patients who received MIRE even without difference in pain intensity. In order to understand the effect on neuropathic pain better, further studies should detail its assessment by using specific scales.
From our knowledge, this is the first systematic review presenting pooled-effects of MIRE for patient-important outcomes. Pain and sensitivity are directly related to quality of life in patients with diabetes4; furthermore, loss of protective foot sensation is associated with increased risk of lower limb ulceration and amputation.5 A former qualitative review42 reported effects of MIRE in these outcomes but included other types of studies. Our systematic review included RCTs identified through an extensive database source without limitation of language and time of publication. In addition, we incorporated a comprehensive assessment of bias of individual studies and quality of evidence for each outcome using GRADE.
LimitationsAlthough we conducted the systematic review following methodological standards, the presented results have some limitations. We did not use specific gray literature databases to search for studies beyond Google Scholar, which may have affected the inclusion of all eligible studies available. Regarding data analysis, although some of the studies had within-subject comparisons (feet were the unit of randomization, not patients), we used the change difference instead of post-intervention values to perform the meta-analysis. This conservative estimate was preferred in order to deal with the baseline differences found in some of the studies.
ConclusionsMIRE may be associated with a short-term improvement in plantar tactile sensitivity not sustained over time and probably related to no effect on neuropathic pain relief. The overall quality of the evidence is low, meaning that further studies are likely to change the estimated effect, not supporting the current use of MIRE in patients with DSPN.
Ethical approvalNot applicable.
FundingCoordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflict of interestThe authors declare no conflicts of interest.
The authors acknowledge Dr. Luis Henrique Canani, for the relevant collaboration on the rationale for this manuscript.