Therapeutic suits or clothing whether associated with intensive protocols or not, became popular in the rehabilitation of children with cerebral palsy. Studies have reported positive effects of these suits on children's posture, balance, motor function and gait. A summary of current literature may help guide therapeutic actions.
ObjectiveTo evaluate the available evidence on the effects of interventions based on the use of therapeutic suits in the treatment of impairments and functional limitations of children with cerebral palsy.
MethodThree independent reviewers searched for experimental studies on MEDLINE, SciELO, BIREME, LILACS, PEDro and CENTRAL databases, between October and December 2015 and updated in May 2016. The reviewers evaluated the methodological quality of selected studies using the Checklist for Measuring Quality. The Grading of Recommendations Assessment, Development and Evaluation was used to synthesize the quality of evidence and strength of recommendation.
ResultsFrom the 13 studies, two evaluated the Full Body Suit, two tested the Dynamic Elastomeric Fabric Orthose, three evaluated TheraTogs and six tested the TheraSuit/AdeliSuit protocols. The quality of evidence for the Full Body Suit, the Dynamic Elastomeric Fabric Orthose and the TheraSuit/AdeliSuit protocols was very low for body structure and function outcomes, while the evidence for TheraTogs was low quality. Regarding the activity outcomes, the Full Body Suit and TheraSuit showed very low quality evidence while the evidence for TheraSuit/AdeliSuit protocols were of low quality.
ConclusionEnthusiasm with new therapeutic approaches that argue modifications in the neuromusculoskeletal impairments and functional limitations of children with cerebral palsy need to be guided by scientific evaluation. The low quality of evidence suggests caution in recommending the use of these therapeutic suits. New studies could change the findings of this review.
Rehabilitation of children with cerebral palsy (CP) has focused on minimizing impairments and disabilities, promoting functioning in patients’ body structures and functions, activity and participation,1 in addition to improving their quality of life.2 New technologies have been used to support and/or enhance the engagement of these children in activities and tasks in different environments.2 One example is the use of rigid and dynamic orthoses. These devices are intended to improve posture and movement, prevent deformities and facilitate functional performance.3
Since the 1990s, different types of therapeutic suits have been used for children with CP.4–6 These suits are dynamic orthoses available in various models. Body suit type orthoses are custom manufactured, made of Lycra, fit tight to the body and may cover the trunk and limbs, exerting a compressive force on the body.4,7 TheraTogs are elastic straps attached by Velcro onto a vest, onto shorts and onto anchors on the legs and feet. These technologies are intended to improve postural alignment, joint stability and movement efficiency.8
TheraSuit, AdeliSuit and PediaSuit were created from a prototype developed for Russian astronauts so they could perform counter-resistance exercises in zero gravity situations.5,9,10 These models have hooks that anchor a system of individually fixed elastic tubes that exert traction between the trunk and pelvis and between the pelvis and lower limbs.9 They have become popular in many countries and are often associated with specific treatment protocols. The TheraSuit Method (TSM) and AdeliSuit Therapy (AST) protocols9,11,12 consist of intensive treatments, including vigorous strengthening and stretching exercises and training of specific motor activities, during which the child wears the suits.9,13
Therapeutic suits have gained popularity in pediatric rehabilitation and are widely commercialized. Families of children with CP have made efforts to acquire these suits and submit their children to these very expensive supplementary treatments.12–16 There are clinical claims that the use of these dynamic orthoses can modify joint alignment and contribute to the strengthening and/or stretching of certain muscle groups, thereby affecting posture, balance, coordination, gross motor function, hand function and gait of children with CP and other health conditions.4–11 The mechanisms of action proposed to explain such functional changes are the compression and/or continuous tension exerted by the suits’ elastic elements on the child's musculoskeletal system. These elastic elements are systematically adjusted based on individual needs and limitations.5,8 In addition to the clinical claims and families’ positive expectations, studies have provided scientific evidence on the effects of these suits regarding posture and movement of children with CP.13,16
A recent systematic review with meta-analysis showed that the effect of TSM and AST protocols on the functioning of children with CP was of small magnitude.13 As this review focused on specific intensive training protocols, which involved elements other than suit wearing, no conclusions regarding the effects of alternative types of therapeutic suits (e.g., TheraTogs) worn by children with CP irrespectively of intensive training can be drawn. Moreover, given that the commercially available interventions include therapeutic suits associated or not with intensive protocols, it is necessary to evaluate the isolated and combined effects of these two elements (i.e., intensive training and suit wearing). It is possible that positive evidence of one element does not reflect the effect of the other, nor the combination of both. Therefore, the objective of this systematic review was to evaluate the available evidence regarding the effects of interventions based on the use of therapeutic suits (combined or not with intensive protocols) on the treatment of functional limitations and disabilities in children with CP. The summary and critical analysis of this literature may guide clinical decision making regarding these resources and provide scientific evidence to enable rehabilitation services to make judicious choices about the provision of these types of treatment.
MethodsThree independent examiners performed literature searches on MEDLINE, SciELO, BIREME, LILACS, PEDro and Cochrane Central Register of Controlled Trials databases. The searches were conducted between October and December 2015 and updated in May 2016. These searchers were standardized and involved no restrictions of year of publication. The search strategy, along with the inclusion and exclusion criteria, is shown in Table 1. As the use of therapeutic suits in child rehabilitation is a relatively new modality of treatment, studies with different experimental designs were included.
Search strategy, inclusion and exclusion criteria for a systematic review on the effects of interventions with therapeutic suits on impairments and functional limitations of children with cerebral palsy.
| Search terms and expressions | Cerebral palsy AND: Lycra garments; |
| TheraSuit; | |
| Compression clothing; | |
| Space suit; | |
| AdeliSuit; | |
| TheraTogs; | |
| PediaSuit; | |
| Suit therapy; | |
| Penguin suit; | |
| Dynamic orthoses | |
| Inclusion criteria | Participants: children and adolescents with cerebral palsy. |
| Type of study design: clinical trial (controlled or not), quasi-experimental, single-case experimental study. | |
| Objective: evaluate the effect of using therapeutic suits on outcomes from the body structure and function and/or activity ICF components. | |
| Language: English, Portuguese or Spanish. | |
| Exclusion criteria | Studies that did not describe the procedures of the therapeutic suit intervention. |
| Studies that did not report the inferential statistics used to analyze the investigated outcomes. | |
ICF, International Classification of Functioning, Disability and Health.
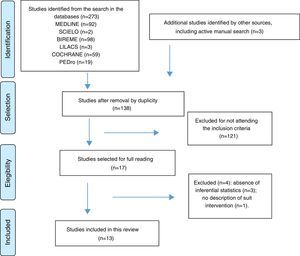
After reading the titles and abstracts, duplicate studies or studies that did not meet the inclusion criteria were excluded. The remainder were selected for full reading. An active manual search on the articles’ reference lists was performed to identify potentially relevant studies. Fig. 1 illustrates the selection process.17
Flowchart illustrating the article selection process, according to the PRISMA17 structure for a systematic review on the effects of interventions with therapeutic suits on impairments and functional limitations of children with cerebral palsy.
The following data was extracted from the selected articles: author and year of publication; study design; sample size and characteristics; details of the intervention: frequency, duration, suit type and settings, activities performed and control intervention; outcomes analyzed using inferential statistics and the instrumentation used; and the results obtained. Due to heterogeneity regarding the therapeutic suits types, intervention protocols and samples’ characteristics across the studies, it was not possible to conduct a meta-analysis.
Each study's methodological quality was evaluated independently by three examiners using the Checklist for Measuring Study Quality,18 which is a valid and reliable instrument recommended to be used in systematic reviews that include studies with different experimental designs.18,19 The checklist evaluates the methodological quality of articles according to the following aspects: reporting (10 items); external validity (3 items); internal validity – bias (13 items); and statistical power (one item).18,19 Twenty-five items are given a score of zero (0) if the study does not meet the requirements or a score of one (1) if the study meets the item requirements. Item 27 on statistical power is scored between zero (0) and five (5), with higher scores for studies with larger samples. Item five, related to principal confounders, is scored between zero (0) and two (2). The maximum possible score is 32.18 Interrater reliability for the use of the checklist has been verified using the Intraclass Correlation Coefficient (ICC) type 2.1, a coefficient that measures absolute agreement with two-way random analysis.20 Consensus regarding disagreements between examiners in the checklist application was reached after a discussion mediated by the first author.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE)21,22 summarized evidence regarding the therapeutic suits effects on each functioning component1 and classified the strength of the recommendation for this therapeutic resource. In GRADE, evidence is initially categorized into four levels (i.e., high, moderate, low, and very low) depending on the study design. Afterwards, several methodological attributes from the studies are taken into account and may cause the level of quality of the evidence available for each outcome to be increased (i.e., large effect size, dose-response gradient, and residual confounding factors that increase the confidence in the estimate) or decreased (i.e., methodological limitations, risk of bias, inconsistency, inaccuracy, indirect evidence and publication bias).21,22 The strength of the recommendation is classified as strong or weak and expresses an indication that a treatment should be adopted in clinical practice or not by considering potential advantages and disadvantages.21,22 The GRADE recommendations were performed by the first author.
ResultsFrom an initial total of 273 articles, 13 met the criteria and were included in this review (Fig. 1). The studies were published between 2000 and 2015. Two studies investigated the effects of the Full Body Suit (FBS), two investigated the Dynamic Elastomeric Fabric Orthose (DEFO), three investigated TheraTogs, and six investigated the TSM or AST protocols. A total of 285 children with different clinical types of CP, aged between three and 17 years, with Gross Motor Function Classification System (GMFCS)23 levels between I and IV, made up the sample. In terms of the designs, six studies were Randomized Controlled Trials (RCTs), five were quasi-experimental designs (QED), and two were single-subject experimental designs (SSED). Interventions with the suits ranged from three to 18 weeks, with usage times ranging between 30min and 12h/day. Different standardized instruments evaluated outcomes focusing on (1) body structures and functions and (2) activity.1 A summary of the studies is presented in Table 2.
Summary of evidence from studies investigating the effects of therapeutic suits associated or not with intensive protocols.
| Author/year | Study design | Sample | Intervention | Variables/instruments | Outcomes |
|---|---|---|---|---|---|
| Matthews et al., 200924 | SSED – ABA. Each phase lasted 6 weeks. | 8 children; 3–13 years; diplegic CP; crouch gait; GMFCS I–III. | DEFO 8h/daily (mean=6.9h), 6 weeks. Usual rehabilitation sessions were maintained. | Gait speed (10-Meter Walking Test). | Five out of 8 children presented a statistically significant improvement in gait speed between phases A1 (baseline) and B (intervention) (p<0.05 – celeration line). |
| Bahramizadeh et al., 201525 | QED. Assessments: EG baseline (without suit) and post-treatment (with suit); CG (single assessment). | EG: 10 children; 5–11 years; diplegic CP; crouch gait; GMFCS I–II. CG: 10 typically developing children, age and weight matched. | EG: DEFO 4–6h/daily, 6 weeks. GC: no intervention. | EG: Knee joint angle in the standing position (electrogoniometer). EG and CG: postural control by means of velocity and displacement of the center of gravity (force plate). | The EG showed a statistically significant reduction in knee flexion in the standing position post intervention compared to baseline (p=0.009), with large effect (d=2.54). No significant differences between groups (p>0.05) were identified for postural control variables. |
| Rennie et al., 200026 | QED. Assessments: baseline (without suit) and post-treatment (with suit). | 7 children with CP; 5–11 years; spasticity, athetosis, hypotonia; able to walk 5m without support. 1 child with Duchenne muscular dystrophy. | Full body suit (Kendall-Camp UK Ltd), 6h/daily, 6 weeks. Usual physiotherapy treatment as well as the use of orthotic devices were maintained during the intervention although neither of them have been described. | Proximal and distal stability during gait (3D-MAS); FS and CA (PEDI) | No significant differences in gait stability or PEDI scores post intervention compared to baseline. |
| Nicholson et al., 200127 | QED. Assessments: baseline (without suit) and post-treatment (without suit). | 12 children; 2–17 years; athetosis, ataxia, spastic, hemiplegic, quadriplegic, diplegic. GMFCS level not informed. | Full body suit (Kendall-Camp UK Ltd), 6h/daily, 6 weeks. Usual rehabilitation treatment as well as the use of orthotic devices were maintained during the intervention although neither of them have been described. | FS and CA (PEDI) | PEDI-FS: significant improvement in self-care (p<0.01) and mobility (p<0.05) post intervention. PEDI-CA: significant improvement in mobility (p<0.05) post intervention. |
| El-Kafy and El-Shemy, 201328 | RCT: 2 groups. Assessments: baseline (without suit) and post-treatment (two conditions: wearing and not wearing the suit for the EG). | 30 children; 6–8 years; diplegic CP; crouch gait; GMFCS I–II. Subjects were randomized in 2 groups: CG (n=15) and EG (n=15). | CG: postural reactions facilitation, postural correction while walking, gait training, 2h/daily, 3 days/week, 12 weeks. EG: same intervention as CG in addition of wearing TheraTogs for 12h/daily (except on weekends). Elastic straps were positioned so as to favor femur's external rotation and tibia's internal rotation. | Rotational angles of the hip and knee in the standing position, Foot progression angle during the gait cycle and gait speed (3D-MAS). | Between groups: statistically significant differences for all the kinematic parameters, favoring the EG (p<0.01), with large effect (1.0<d<1.91). Within-groups: CG: no significant differences post-treatment (p>0.05); EG: significant differences between the three assessments for all the kinematic parameters; the “wearing suit” condition was the one in which participants showed the best results (p<0.01), with large effect CG vs EG “wearing suit” (2.79<d<3.98). |
| El-Kafy, 201429 | RCT: 3 groups. Assessments: baseline (without suit) and post-treatment (without suit). | 51 children; 6–8 years; diplegic CP; crouch gait; GMFCS I–II. Subjects were randomized in 3 groups: CC (n=18), EG1 (n=16), EG2 (n=17). | CG: NDT, 2h/daily, 5 days/week, 12 weeks. EG1: NDT in addition of wearing TheraTogs 12h/daily (elastic straps were positioned so as to favor femur's external rotation and tibia's internal rotation). EG2: same as EG1 in addition of wearing GRO during treatment sessions. | Gait kinematics: hip and knee flexion during the stance phase; gait speed; cadence; step length. Rotational angles of the hip and knee during the stance phase were assessed in EG1 and EG2 (3D-MAS). | Between groups: statistically significant differences between groups for all the kinematic parameters, favoring the EG2 (p<0.05), with small to large effect CG vs EG1 (0.25<d<1.48) and large effect CG vs EG2 (1.03<d<3.10). EG1 vs EG2: no significant differences between groups for the rotational angles of hip and knee (p>0.05). Within-groups: significant improvements in all kinematic parameters were observed in the three groups (p<0.05), with large effect (0.97<d<4.96). |
| Flanagan et al., 200930 | QED. Assessments: baseline, post-treatment (wearing and not wearing the suit), follow-up (2 and 4 months after intervention – no suit). | 5 children; 7–13 years; diplegic CP; GMFCS I. | TheraTogs 10–12h/daily, 12 weeks. Individual suit adjustment. All subjects received elastic straps for oblique and erector spinae muscles. Participants did not receive other therapies during the intervention period. | Gait kinematics (3D-MAS); gross motor abilities and balance (BOTMP); performance and satisfaction perceived by the caregiver regarding the performance of functional tasks (COPM). | Gait kinematics: significant improvement in peak hip extension and pelvis alignment post-treatment wearing the suit vs the other assessment conditions. No changes in gait speed, cadence or step length were identified. BOTMP: significant difference between baseline and post-treatment not wearing the suit (p=0.025) moderate effect (d=0.76), post-treatment wearing the suit (p=0.023) large effect (d=0.89), follow-up 2 months (p=0.007) moderate effect (d=0.70) and follow-up 4 months (p=0.02) large effect (d=0.96). COPM: no significant improvement with the exception of satisfaction after follow-up 2 months. |
| Alagesan and Shetty, 201131 | RCT: 2 groups. Assessments: baseline and post-treatment. | 30 children; 4–12 years; diplegic CP. Subjects were randomized in 2 groups: CG (n=15) and EG (n=15). | CG: conventional therapy (active limb movements, muscle strengthening and stretching, weight bearing and shifting, orthostatic posture training, abnormal posture corrections, balance training, gait training and stair climbing training), 2h/daily, 5 days/week, 3 weeks. EG: same as CG in addition of wearing TheraSuit. | Gross motor function (GMFM-88). | Between groups: significant differences in GMFM-88 scores between groups, favoring the EG (p=0.03) with large effect (d=0.83). Within-groups: both groups showed significant improvement in GMFM-88 scores after intervention (p<0.001), with small effect to CG (d=0.11) and small effect to EG (d=0.4) |
| Bailes et al., 201132 | RCT. 2 groups. Assessments: baseline, post-treatment (3–4 weeks), follow-up (4 weeks). | 20 children; 3–8 years; GMFCS III. Subjects were randomized in 2 groups: CG (n=10) and EG (n=10). | EG: TheraSuit Method, 4h/daily, 5 days/week, 3 weeks. Subjects in EG wore TheraSuit with the elastic bungee cords attached. CG: same intervention method as EG, although subjects in this group wore a “control suit” (TheraSuit without the elastic bungee cords attached). | Gross motor function (GMFM-66). FS and CA (PEDI). | Between groups: no significant differences between groups neither in GMFM-66 scores (p=0.48), nor in PEDI subscales scores (p>0.18); the combined effect, although of small magnitude, was positive for all the outcome variables (0.17<d<0.23), except for PEDI-CA mobility, which was negative (d=−0.37). Within-groups: EG significant improvement in PEDI-CA self-care post-treatment vs baseline (p=0.04) small effect (d=0.19); significant improvement in GMFM-66 scores (p=0.002) small effect (d=0.37), PEDI-FS self-care (p=0.04) and mobility (p=0.005) small effect (0.20<d=0.25), PEDI-CA self care (p=0.01) small effect (d=0.24) in the follow-up vs baseline; PEDI-FS mobility in the follow-up vs post-treatment (p=0.03) small effect (d=0.14). CG: significant improvement in GMFM-66 scores in the follow-up vs baseline (p=0.03) moderate effect (d=0.54). |
| Bar-Haim et al., 200633 | RCT: 2 groups. Assessments: baseline, post-treatment (4 weeks), follow-up (9 months). | 24 children; 5–12 years; hemiplegic, quadriplegic, triplegic CP; GMFCS II–IV. Subjects were randomized in 2 groups: EG (n=12) and CG (n=12). | EG: Adelisuit Therapy, 2h/daily, 5 days/week, 4 weeks. CG: NDT 2h/daily, 5 days/week, 4 weeks. Participants did not receive other therapies during the intervention period. | Gross motor function (GMFM-66). Energy cost during stair-climbing (MEI). | Between groups: no significant differences between groups. Within-groups: EG – significant improvement in GMFM-66 scores (p<0.03) small effect (d=0.24) post-treatment vs baseline, MEI (p<0.05) with large effect (d=1.53) in the follow-up vs baseline, especially in children with higher GMFM-66 scores. CG – significant improvement in GMFM-66 scores in the follow-up vs baseline (p=0.006) with moderate effect (d=0.62). |
| Mahani et al., 201134 | RCT. 3 groups. Assessments: baseline, post-treatment (4 weeks), follow-up (16 weeks). | 36 children; mean age=7.78 years; diplegic, quadriplegic, spastic and dystonic CP; GMFCS I–IV. Subjects were randomized in 3 groups: CG (n=12), EG-AST (n=12) and EG-MAST (n=12). | CG: NDT (passive exercises in the first hour and active exercises in the second hour). EG-AST: AdeliSuit Therapy. EG-MAST: Modified AdeliSuit Therapy (NDT in the first hour and AdeliSuit in the second hour). Subjects in all 3 groups were treated for 4 weeks (2h/daily, 5 days/week). | Gross motor function (GMFM-66). | Between groups: significant differences (p=0.000); the differences were between EG-MAST and EG-AST favoring the EG-MAST (p=0.000) small effect (d=0.41); and between EG-MAST and CG favoring the EG-MAST (p=0.000) moderate effect (d=0.56); no significant differences between EG-AST and CG (p=0.272). Within-groups: the 3 groups improved in GMFM-66 scores post-treatment vs baseline (p<0.001) with small to large effect (0.29<d<0.88); no significant differences within-groups were identified in the follow-up (p=0.637). |
| Christy et al., 201235 | QED. Assessments: baseline, post-treatment (3 weeks), follow-up (3 months). | 17 children; 4–12 years; spastic, hypotonic, athetosis, ataxia, quadriplegic, diplegic and triplegic CP; GMFCS I–III. | TheraSuit Method, 4h/daily, 5 days/week, 3 weeks. | Gross motor function (GMFM-66). Performance in community walking (SAM). Global functionality (PODCI). Performance and satisfaction perceived by the caregiver regarding the performance of functional tasks (COPM). | Post-treatment vs baseline: significant improvement in GMFM-66 scores (p<0.001) small effect (d=0.24), COPM (p<0.001) large effect (2.25<d<2.83) and PODCI (p=0.001) small effect (d=0.45). Follow-up vs baseline: significant improvement in GMFM-66 scores (p=0.01) small effect (d=0.21) and COPM (p<0.001) large effect (1.74<d<2.15). No significant differences in community walking performance (SAM). |
| Ko et al., 201436 | SSED – AB. Phase A: 6 weeks; Phase B: 18 weeks. | 1 child; 8 years; diplegic CP; crouch gait; GMFCS III. | Phase A: baseline. Phase B (intervention): AdeliSuit Therapy, 50min sessions, 1 day/week, 18 weeks. Each session was divided into 10min preparation, 10min muscle strengthening and 30min gait training with AdeliSuit on. Usual physical and occupational therapies were maintained twice a week. | Gait speed (10-Meter Walking Test). Gross motor function (GMFM-88). Balance (PBS). | There was significant improvement in gait speed (p=0.014), GMFM-88 (p=0.012) and PBS scores (0.001) in phase B compared to baseline (two-standard deviation band method). |
SSED, single-subject experimental design; CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; DEFO, Dynamic Elastomeric Fabric Orthoses; QED, quasi-experimental design; EG, experimental group; CG, control group; 3D-MAS, three-dimensional motion analysis system; PEDI, Pediatric Evaluation of Disability Inventory (FS, Functional Skills Scale; CA, Caregiver Assistance Scale); BOTMP, Bruininks-Oseretsky Test of Motor Proficiency; COPM, Canadian Occupational Performance Measure; RCT, randomized controlled trial; NDT, neurodevelopmental treatment; GRO, ground reaction orthosis; GMFM, Gross Motor Function Measure; MEI, Mechanical Efficiency Index; SAM, Step Watch Activity Monitor; PODCI, Pediatric Outcomes Data Collection Instrument; PBS, Pediatric Balance Scale.
Two studies tested the effects of DEFO (neoprene pants that exert pressure on the pelvis and promote hip external rotation and abduction and knee extension)24,25 on children with diplegic CP presenting with a crouch gait. In both studies, subjects wore the suit from four to eight hours a day for six weeks. Matthews et al.24 showed that five of the eight subjects significantly increased walking speed after intervention compared to baseline. The fact that only some of the participants showed improvements might be attributed to (1) the lack of control of the activities performed by each participant during the intervention period and (2) the lack of standardization regarding suit usage time among children.24 Bahramizadeh et al.25 showed improvement in knee alignment in the standing position, in the DEFO wearing assessment condition, after the intervention period. Wearing this suit did not lead to significant difference in postural control between CP and normally developing children.25
The Full Body SuitTwo studies, conducted by the same group of researchers, investigated the effects of the FBS.26,27 In these studies, children wore the suit for up to six hours/day for six weeks while maintaining their usual treatments and orthoses.26,27 Nicholson et al.27 revealed significant differences in self-care and mobility skills and in mobility independence, whereas Rennie et al.26 found no effect on any Pediatric Evaluation of Disability Inventory (PEDI) scales nor on proximal and distal stability during walking. It is possible that the discrepancy in their results might be attributed to type II error, wherein one study's small sample size26 precluded demonstration of the effects that were shown in the other study.27
TheraTogsThree studies tested the effects of TheraTogs in children with diplegic CP wearing the suits for 12h/day for 12 weeks.28–30 El-Kafy and El-Shemy28 compared a control group (CG) submitted to an exercise program, with an experimental group (EG) who undertook the same program in addition to wearing TheraTogs. Their results showed a significant difference between groups for postural alignment and gait kinematics, favoring the EG. Within-groups comparison revealed that CG did not improve after treatment. In addition, after the intervention, evaluation of the EG wearing the suit revealed significant improvements in all parameters compared to the conditions without the suit evaluated before and after intervention.28 El-Kafy29 compared the following three groups: CG – neurodevelopmental treatment (NDT); EG1 – NDT plus TheraTogs; and EG2 – NDT plus TheraTogs and ground reaction orthosis. After the interventions, although the three groups showed improvements in all gait kinematic parameters, EG2 showed better results than CG and EG1, demonstrating that combining rigid and dynamic orthoses might potentiate gait performance in diplegic children with a crouch gait.29 Flanagan et al.30 demonstrated a positive effect of TheraTogs on gross motor skills and balance after intervention and at follow-up. Changes in gait kinematics were found with the suit on (i.e., increase in peak hip extension and pelvic alignment). Even after prolonged use, the effects of TheraTogs were demonstrated only when children were wearing the suit.30
TheraSuit Method and AdeliSuit Therapy ProtocolsTwo studies31,32 investigated the effects of the TheraSuit associated with intensive protocols on activity outcomes1 of children with CP. In both studies, participants were submitted to exercise programs, with one group (EG) wearing the suit and the other group (CG) not wearing it. Alagesan and Shetty31 found a significant improvement in gross motor function in the EG compared to the CG. Bailes et al.32 found improvements in gross motor function, functional abilities and caregiver assistance in both groups but found no difference between CG and EG post-treatment.32 Although both studies showed significant effects following the use of this suit, there were inconsistencies in groups’ comparisons due to differences in the treatment characteristics. For example, in the study conducted by Alagesan and Shetty31 the exercise program was based on conventional therapy, whereas Bailes et al.32 used the original TSM protocol.
Two studies33,34 compared AST to NDT and found no difference in gross motor function in groups of children with different CP types. Mahani et al.34 also compared NDT and AST to the modified AST (MAST) protocol, which associates NDT and AST techniques. The group submitted to MAST showed better results in gross motor function, revealing the positive effects of combining a traditional technique with the use of this therapeutic suit.34 Christy et al.35 investigated the effects of TSM on 17 children with CP and found a significant improvement in gross motor function, overall functioning, performance and satisfaction perceived by caregivers regarding children's performance of functional tasks.35 Ko et al.36 evaluated the effects of AST in a child with diplegic CP undergoing weekly sessions for 18 weeks. Improvements in gross motor function, gait speed and balance were observed.36
Evaluation of the methodological quality of the studiesThe consistency between the three researchers regarding the use of the Checklist for Measuring Quality for measuring study quality18 was very good20 (ICC2.1=0.89±0.09). Table 3 shows the scoring for each study for each item. Scores varied according to the design, with higher values obtained by RCTs,29–34 followed by QEDs25–28,35 and SSEDs.24,36 Studies analyzing the effects of FBS obtained scores of 37%26 and 40%,27 studies of DEFO obtained 43%24 and 53%,25 TheraTogs studies obtained 37%,28 59%29 and 68%,30 TSM and AST studies31–36 scored between 34% and 90% of the total checklist value. Overall, the studies obtained good scores in the description of objectives, methods and results. Low scores were related to external validity items and the blinding of subjects, therapists and examiners. The item regarding consistency in the intervention was scored only by studies that verified the effects of TSM and AST; studies evaluating the effects of FBS, DEFO and TheraTogs received a zero score in this item because they did not provide information about the control of suit usage time. Most studies obtained a maximum score on the item related to statistical power (score 5), because study groups had eight or more subjects, leading to the checklist’ criteria for maximum scoring on that item.18
Evaluation of the methodological quality of studies on the effects of interventions with therapeutic suits on impairments and functional limitations of children with cerebral palsy using the Checklist for Measuring Qualitya.
| Checklist items | Selected studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Matthews et al.24 | Bahramizadeh et al.25 | Rennie et al.26 | Nicholson et al.27 | El-Kafy and El-Shemy28 | El-Kafy29 | Flanagan et al.30 | Alagesan and Shetty31 | Bailes et al.32 | Bar-Haim et al.33 | Mahani et al.34 | Christy et al.35 | Ko et al.36 | |
| Reporting | |||||||||||||
| 1. Hypothesis/aim/objective | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Inclusion/exclusion criteria | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4. Interventions | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5. Principal confounders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| 6. Findings | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7. Random variability | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8. Adverse events | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| 9. Lost to follow-up | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| 10. Probability values | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| External validity | |||||||||||||
| 11. Subjects asked to participate representative of population | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12. Subjects prepared to participate representative of population | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13. Representative of the treatment | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Internal validity | |||||||||||||
| 14. Blinding subjects to the intervention | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 15. Blinding of examiners | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| 16. Clear “data dredging” | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| 17. Adjust for different lengths of follow-up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| 18. Appropriate statistical tests | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19. Reliable compliance with the intervention | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| 20. Accurate outcome measures | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 21. Recruitment population of subjects | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 22. Recruitment period of time of the subjects | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 23. Randomization groups | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 24. Concealed randomized intervention assignment from patients and staffs | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25. Adjustment for confounding | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 26. Losses of subjects to follow-up | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| Power | |||||||||||||
| 27. Sufficient power to detect a clinically effect/sample sizes | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 5 | 5 | 1 |
| Study total score | 14 | 17 | 12 | 13 | 19 | 22 | 12 | 20 | 29 | 23 | 24 | 19 | 11 |
| Percentage (%)b | 43 | 53 | 37 | 40 | 59 | 68 | 37 | 62 | 90 | 71 | 75 | 59 | 34 |
The summarized evaluation by GRADE suggests that the quality of evidence on the use of the suits was low to very low for body structure and function and activity1 outcomes. Such classification was primarily due to the small number of RCTs and to certain methodological limitations in the studies, resulting in weak recommendations for the use of therapeutic suits. Table 4 shows the application of GRADE with respect to the body of evidence available for the outcomes investigated for each suit.
Evidence profile of the four models of therapeutic suits and intensive protocols associated with therapeutic suits.
| Suits | ICF-level outcome | Studies | Participants | Outcome variables | Comments | Quality of evidence | Recommendation |
|---|---|---|---|---|---|---|---|
| DEFO | Body structure and function | Bahramizadeh et al.25 | 10 children with CP | Postural alignment and control | Important methodological limitations | Very low | Weak |
| Activity | Matthews et al.24 | 8 children with CP | Gait velocity | Important methodological limitations | Very low | ||
| Full Body Suit | Body structure and function | Rennie et al.26 | 7 children with CP | Gait stability | Effect was not reported | Very low | Strong (–) |
| Activity | Rennie et al.26 and Nicholson et al.27 | 19 children with CP | Functional skills, caregiver assistance | Important methodological limitations, results inconsistency | Very low | ||
| TheraTogs | Body structure and function | El-Kafy and El-Shemy28, El-Kafy29 and Flanagan et al.30 | 86 children with CP | Gait kinematics, postural alignment | Methodological limitations | Low | Weak |
| Activity | El-Kafy29 and Flanagan et al.30 | 56 children with CP | Gait velocity, gross motor function, perceived satisfaction and performance | Methodological limitations | Low | ||
| TheraSuita | Activity | Alagesan and Shetty31 and Bailes et al.32 | 50 children with CP | Gross motor function, functional skills, caregiver assistance | Methodological limitations, results inconsistency | Very low | Weak |
| TheraSuit Method/AdeliSuit Therapy | Body structure and function | Bar-Haim et al.33 and Ko et al.36 | 25 children with CP | Energy cost, gait velocity, balance | Methodological limitations, indirect evidence | Very low | Weak |
| Activity | Bailes et al.32, Bar-Haim et al.33, Mahani et al.34, Christy et al.35 and Ko et al.36 | 128 children with CP | Gross motor function, functional skills, caregiver assistance, global function, perceived satisfaction and performance | Methodological limitations, results inconsistency | Low | ||
ICF, International Classification of Functioning, Disability and Health; DEFO, Dynamic Elastomeric Fabric Orthoses; CP, cerebral palsy.
This systematic review examined the available evidence on the effects of therapeutic suits associated or not with intensive protocols on the functioning of children with CP. Four main suit models were found in the 13 selected studies. Generally, the postural alignment and gait kinematic improved in children with CP who wore the suits, especially when they were wearing them. However, the quality of evidence is low and the recommendation for therapeutic suits in the treatment of impairments and limitations of children with CP is weak due to uncertainty regarding the advantages and disadvantages of wearing the investigated suits.
The review did not find any effect arising from the isolated use of TheraSuit and AdeliSuit without an associated intensive treatment protocol in the functioning of children with CP. The association of the suits with intensive protocols (e.g., TSM/AST) has increased expectations of good results in the rehabilitation of children and youth with CP.2,12,14,15 Among the selected studies, Bailes et al.,32 with better methodological quality, showed that there was no difference in the gross motor function and functional skills of children with CP when submitted to the TSM with or without the suit. The effects found might be due to the intensity of treatment and cannot be attributed to the isolated use of TheraSuit.32 In addition, Bar-Haim et al.33 and Mahani et al.34 revealed no difference between those interventions and other therapies when performed with the same intensity and duration. It is possible that the high intensity of training, instead of the specificity of the TSM and AST protocols, may be responsible for the improvements presented by the children submitted such interventions. This review corroborates a recent systematic review and meta-analysis13 that examined the effects of these therapies on the functioning of children with CP. The authors concluded that the available evidence was not sufficient to draw conclusions about the advantages and disadvantages of the TSM and AST in the treatment of gross motor function of children with CP.13
The DEFO and TheraTogs demonstrated positive effects24,25,28–30 on postural alignment and gait kinematics of children with diplegic CP. These individuals often present hip internal rotation and flexion, knee flexion, and tibia internal rotation, resulting in a gait pattern named “crouch gait”.37 Positive effects on the posture and gait of these children were previously documented with subjects wearing rigid orthoses.3,38 Hence, the association of rigid orthoses with a therapeutic suit (i.e., a dynamic orthoses) seems to potentiate the individual effects of each of these interventions in the diplegic children's gait kinematics.30 While rigid orthoses provide greater joint stability, dynamic orthoses have the potential to facilitate the execution of movements and their combination may perpetuate long-term positive changes in the musculoskeletal system of diplegic children.39,40
FBS, whose compressive characteristic aims to promote stability, did not demonstrate any improvements in gait stability when used in children with different CP types, even when the child was wearing the suit during the assessments.26,27 Additionally, Rennie et al.26 and Nicholson et al.27 have reported parents’ complaints concerning the discomfort associated with FBS, including changes in the urinary system and constipation, movement restrictions, warmth and eczema. These discomforts contributed to most children not enjoying the use of the suit and reporting they would not consider wearing it again.26,27 Knox41 followed a series of eight cases of children with CP who wore a suit with the same compressive characteristics for four weeks. Three children withdrew from participating in the study because they could not adapt to the suit. Those who completed the intervention stated that the suit was tight, hot, and difficult to put on and take off, with difficulties in using the toilet.41 The very low evidence of FBS's efficacy, in association with its adverse effects, result in a strong recommendation that it should not be used in the rehabilitation of children with CP.
The intensity and duration of therapeutic suit treatment varied greatly among the studies. Positive effects on body structure and function outcomes were found in situations of reduced intensity and duration25 (i.e., wearing DEFO for four to six hours/day for six weeks) as well as in situations of higher intensity28–30 and duration (i.e., wearing TheraTogs for 12h/day for 12 weeks). However, long-term effects were not systematically investigated. Only Flanagan et al.,30 investigated the isolated effects of TheraTogs, and found stable gains in gross motor skills in the follow-up period.30 Due to the differences among therapeutic suits and the time regimens in which they were implemented, the available evidence is not conclusive regarding the optimal intensity of suit wearing to guarantee their efficacy.
This systematic review has limitations that need to be considered. The criteria for selecting studies was met with a language restriction (only studies written in English, Portuguese or Spanish were included), resulting in the exclusion of seven potentially relevant studies published in Russian. Besides that, no literature searches were conducted in databases with no free access (e.g., EMBASE), which may have hindered the identification of potentially relevant studies. The Checklist for Measuring Study Quality presented difficulties regarding the interpretation and application of some items, especially those concerning external validity, which caused disagreements between the evaluators. These disagreements were minimized by discussion to reach a consensus. Finally, it was not possible to add a meta-analysis to this systematic review because the studies tested various therapeutic suit models in samples with different characteristics and compared different protocols.
Recommendations for future studiesThe selected studies did not have sufficient information about (1) the direction and level of tension applied to the elastic elements in order to adjust the suits and (2) the exercises and activities conducted during the therapy sessions. Detailing the procedures is important since these elements constitute the mechanisms underlying therapeutic changes. Additionally, there is a lack of studies with a high level of evidence that identified the dose-response of this therapeutic resource (i.e., the sufficient and appropriate intensity and duration of suit wearing for the effects on different functional components). Finally, the studies lacked information about which children with CP (i.e., descriptive characteristics including age, topographic and severity) might be better candidates for obtaining the effects of different therapeutic suit models. Most studies that were part of this review included only children with mild to moderate severity of CP (GMFCS I–III) and with diplegic CP type, limiting the results for these subgroups.
ConclusionThe suits DEFO and TheraTogs seem to improve the postural alignment and gait performance in children with diplegic CP. However, the quality of current available evidence ranges from low to very low for the different suit models tested. The recommendation to use these suits in the treatment of children with CP is weak. Future studies that address the inconclusive elements indicated in this review may help to endorse or refute the effects of these dynamic orthoses and will most certainly affect the strength with which they can be recommended.
Conflicts of interestThe authors declare no conflicts of interest.
Financial support was granted by the Brazilian government agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), and Laboratório de Investigação Médica do Hospital das Clínicas da FMUSP – LIM 34 (Ciências da Reabilitação). Funding from CNPq (Universal 14/2012 – Faixa B; Process: 477575/2012-9) paid for the English translation.