Cerebral palsy (CP) is one of the main causes of disability in childhood. Virtual reality (VR) has been used as a treatment option in this population, however its effectiveness is unclear.
ObjectiveTo evaluate the effectiveness of VR in patients with CP.
MethodsWe conducted electronic searches in EMBASE, MEDLINE, Cochrane library, PEDro, AMED, PsycoINFO, and LILACS databases and trial site registries such as ClinicalTrials.gov and ICTRP. We included randomized controlled trials that tested the use of VR alone or in combination with other interventions compared to more conventional rehabilitation or usual care in individuals with CP. The primary outcomes were upper and lower limb function, postural control, and balance. The secondary outcomes included global motor function, perception, cognition and spatial functions, motivation, motor learning, and adverse events. Two independent reviewers extracted and assessed included articles for risk of bias using the Cochrane risk of bias tool. We use a meta-analysis with random effect model whenever possible. We analyzed the quality of evidence using theGRADE approach.
ResultsWe included 38 trials (pooled n = 1233 participants) in this review. There is very low quality of evidence that VR plus conventional rehabilitation is better than conventional rehabilitation for upper limb function. There is also very low quality evidence that VR alone is no better than conventional rehabilitation for upper and lower limb function. No adverse events were observed among the 10 trials that provided information on this outcome.
ConclusionAt present we have very limited to limited confidence in effect estimation for utilization of VR in this population. Future studies may change our confidence in results and effect estimates.
Protocol registrationPROSPERO CRD 42018102759.
Cerebral palsy (CP) is one of the main causes of physical disability in children worldwide.1,2 Children with CP have altered motor function and postural control, which are highly associated with limitations in daily life activities.1,3,4,5 The prevalence of CP over the last 40 years is estimated to have increased from 1.5 to 2.5 per 1000 newborns.6
Virtual reality-based interventions (VR) have been used in the treatment of patients with CP.7,8 VR can be defined as an interactive immersion and simulation experience in an environment and real-world objects created by a computational system responding to real-time user movements.9,10 VR can be offered in an immersive (i.e. head-mounted display) and non-immersive (i.e. desktop display) manner. Rehabilitation with VR enables training at different levels of complexity with feedback in a safe and controlled environment.11,12 In addition, VR can enable clinicians to control the duration and intensity of tasks not performed in the real world.13 At the same time, VR offers meaningful learning experience by providing encouragement and motivation during rehabilitation.11
Recently, randomized controlled trials were conducted to analyze the effects of VR in several areas.14,15,16,17,18,19,20 A 2017 systematic review21 assessed the effectiveness of VR rehabilitation on sensory and functional motor skills of children and adolescents with CP. A limitation of this review was the inclusion of study designs other than randomized controlled trial (e.g. case studies).21 A 2018 systematic review22 was the first comprehensive review with meta-analysis including only randomized controlled trials.22 However, there has been several recent studies published since their search in 2016.23,24,25,26,27,28,29,30,31 A 2019 systematic review assessed the effectiveness of VR in children with CP but only for hand function.32 Furthermore, the previous systematics reviews do not provide the strength and quality of the evidence (e.g. GRADE approach) based on VR applied in isolation or in combination with other treatments and for a wide range of outcomes. Thus, an updated and more comprehensive systematic review is warranted. We aimed to systematically review the effectiveness of VR to improve upper and lower limb function, postural control, and balance compared to other type of treatments, usual care, waiting list, no treatment or placebo in children and young adults with CP.
MethodsProtocol and registrationThis review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) CRD: 42018102759. This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (The PRISMA Statement).89
Study selectionWe included randomized controlled trials that consisted of individuals with CP. CP was defined as a group of non-progressive neurological disorders of motor and postural development that occurs in fetal or infant brain development.1 It can be diagnosed by clinical examination and/or imaging tests. There were no exclusions regarding age, CP types, location of motor change, and severity.
We included the following types of VR: video games commercially available (e.g. Nintendo Wii) that were non-immersive or immersive (custom interface); applied in isolation or combined with other treatments. We considered any other type of treatments, no treatment, waiting list, usual care, or placebo as control groups.
The primary outcomes analyzed were upper limb function, lower limb function (divided into gait and strength of the lower limbs), and postural control and balance. The secondary outcomes included were global motor function, spatial functions, perception and cognition, motivation, motor learning, and adverse events. All outcomes needed to be assessed by a validated instrument. We included four time-points for each outcome: post intervention, short (> two weeks but ≤ three months), intermediate (> three months but ≤12 months), and long term follow-up (> 12 months).
Data sources and searchesThe searches were performed in the following electronic databases: EMBASE (Ovid), MEDLINE (Ovid), LILACS, Cochrane library, PEDro, AMED (Ovid), and PsycINFO (Ovid) in March 2020. There were no restrictions regarding year of publication or language. The search strategy used in the databases is described in the Supplemental online material. Conference proceedings were excluded.
We also performed searches on clinical trial site registries such as ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (www.who.int/ictrp/en/) and manual searches on reference lists of eligible trials. Two authors independently screened the study titles, abstracts, and full texts of all identified studies. Any disagreement among the reviewers was resolved by consensus or arbitration by a third author.
Data extraction and risk of bias assessmentOne author performed data extraction using a standardized form. Extracted data included name of the authors, country, year of publication, type of PC, outcome measures, age, number of participants, VR type, description of interventions, duration of sessions, frequency of intervention, duration of the intervention, and place of recruitment. Finally, data on treatment effects and number of participants in each group were extracted. Attempts to contact the authors via e-mail were made when the information from the studies was insufficient or unclear. We extracted data from graphs using the WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/). Data reported as mean and range of values were converted to mean and standard deviation data.33 If possible, cross-over studies, when identified, had their data extracted preferably at the end of the first phase, if not possible the data were extracted at the end of the last phase and provided in descriptive form.34
We used the 7-criteria Cochrane risk of bias tool.35 Two independent reviewers (JF and RS) analyzed the risk of bias of all included trials. Disagreements between the reviewers were resolved by consensus or arbitration by a third author.
Data synthesis and analysisWe used mean differences (MD) with 95% confidence intervals (CI) when studies used continuous outcomes variables with the same scale. When different scales were used for continuous outcomes, we opted to use standardized mean differences (SMD) with 95% CI. We followed a system commonly used by Cohen 198836 for interpretation of effect sizes: d = 0.2 represents a small; d = 0.5 a medium; and d = 0.8 a large effect, and used in previous studies.37,38Odds ratios with 95% CI were used to calculate treatment effects or adverse events for dichotomous variables. Meta-analyses were performed using Review Manager 5.3.39 When data could not be included in a meta-analysis, data are presented descriptively.
Heterogeneity of data was evaluated by visual inspections of the forest plot, observing the overlap of the 95% CIs, followed by a Chi-square test and I2 statistic as recommended by the Cochrane Handbook.34 We pooled the data using a random effect model. When we observed substantial data heterogeneity, I2> 50% and p < 0.10, quality of evidence was downgraded due to inconsistency. Where possible, we stratified the analyses into subgroups to verify if the effects varied based on the intervention duration (i.e. ≤ 30 minutes, 30–60 minutes, >60 minutes) or frequency of intervention (≤ 2× week, 3× week, ≥ 3× week). We performed sensitivity analyses for VR type (commercial game or personalized game) for comparisons that presented with moderate level of heterogeneity.
We evaluated the quality of evidence for each outcome using GRADE as recommended by the Cochrane Handbook.34 The quality of evidence was based upon the performance of the studies for five domains: 1) study design and risk of bias (downgraded by one level if 25% or more of the participants in the comparison are from studies with high risk of bias defined as one or more criteria classified as high risk of bias in the study); 2) Inconsistency (downgrade by one level if by visual inspection the presence of wide variation of effect estimates is verified or the I2 test is greater than 50%); 3) Indirectness (downgrade by one level if more than 50% of participants vary from the population of interest (e.g. mixed populations or multiple disorders)); 4) Inaccuracy (downgrade by one level if, in dichotomous outcomes, the sample size is less than 300 participants or for continuous outcomes, less than 400 participants40); and 5) Publication bias (downgrade by one level if publication bias was identified by visual inspection of funnel plots if more than 10 studies are included in the comparison). The quality of evidence was defined as follows:
High quality of evidence: New research is unlikely to change confidence in the effect estimates;
Moderate quality of evidence: Further research is likely to have an important impact on the confidence in the effect estimates and may change the estimate;
Low quality of evidence: More research is very likely to have impact on the confidence in the effect of the estimate and is likely to change the estimate;
Very low quality of evidence: There is much uncertainty about the effect of the estimate;
No evidence: No randomized controlled trials were identified to evaluate this outcome.
The primary findings of this review are included in a "Summary of findings" custom table using GRADEpro GDT.41
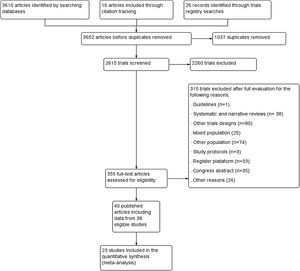
ResultsWe included 38 randomized controlled trials (40 references), of which three had a crossover design (Fig. 1). In total, 1233 participants of both sex with ages between 4 and 38 years old were included. The sample size ranged from 10 to 102 participants with a mean ± standard deviation of 34.2 ± 17.5 participants. The characteristics of included studies are presented in Table 1.
Characteristics of virtual reality type, intervention, and location of included trials.
| Author/ Year | Participants characteristics | Intervention | Duration of Session | Frequency | Duration of Intervention |
|---|---|---|---|---|---|
| Aran et al. 201923 | Country: Turkey; N=90; Mean age 11.1 years old; Type of CP: Hemiplegia | Microsoft Kinect for PC; EG: VR and Traditional Occupational Therapy; CG: Traditional Occupational Therapy | 45 min | 2× week | 10 weeks |
| Arnoni et al. 201924 | Country: Brazil; N=15; Mean age:(years old) 9.6; Type of CP: Mild spastic hemiplegia | Xbox Kinect; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 45-50 min 45 min | 2× week | 8 weeks |
| Ballester et al. 201925 | Country: Spain; N = 14; Mean age:(years old): 38; Type of CP: Tetraplegia, triplegia, diplegia, and hemiplegia | Rehabilitation Gaming System; EG: VR; CG: Conventional Rehabilitation | 30 min | 3× week | 4 weeks |
| Okmen et al. 201926 | Country: Turkey; N = 41; Mean age:(years old): 8.5; Type of CP: Quadriplegia, diplegia, triplegia, and hemiplegia | Eye Toy-Play system (Play station 2); EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 60 min | 3-5× week | 4 weeks |
| Pin and Butler 201927 | Country: China; N = 18; Mean age:(years old): 9.2; Type of CP: Bilateral spastic | TYMO; EG: VR and Usual Rehabilitation Program; CG: Usual Rehabilitation Program | 20 min | 4× week | 6 weeks |
| Pourazar et al. 201928 | Country: Iran; N = 20; Mean age:(years old): 9.4; Type of CP: Spastic hemiplegia | Xbox Kinect; EG: VR; CG: Common Therapy Program | 85-100 minutes | 5× first two week 3× following week 2× last two week | 6 weeks |
| Author/ Year | Participants characteristics | Intervention | Duration of Session | Frequency | Duration of Intervention |
|---|---|---|---|---|---|
| Sahin et al. 201930 | Country: Turkey; N = 60; Mean age:(years old): 10.2; Type of CP: Hemiplegia | Microsoft Kinect; EG: VR and Traditional Occupational Rehabilitation; CG: Traditional Occupational Rehabilitation | 45 min | 2× week | 8 weeks |
| Tarakci et al. 201931 | Country: Turkey; N = 30; Mean age:(years old): 11; Type of CP: Spastic and dyskinetic | Leap Motion Controller; EG: VR; CG: Conventional Rehabilitation | 60 min | 3× week | 8 weeks |
| El-Shamy and El-Banna 201848 | Country: Egypt; N = 40; Mean age:(years old): 9.6; Type of CP: Spastic hemiplegia | Nintendo Wii; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 40-60 min 60 min | 3× week | 12 weeks |
| Sajan et al. 201750 | Country: India; N = 20; Mean age:(years old): 11.5; Type of CP: Diplegic, triplegic, and spastic quadriplegic | Nintendo Wii; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 45 min | 6× week | 3 weeks |
| Gatica-Rojas et al. 201764 | Country: Australia; N = 32; Mean age:(years old): 10.7; Type of CP: Hemiplegia and spastic diplegia | Wii™ balance board; EG: VR; CG: Conventional Rehabilitation | 30 min 40 min | 3× week | 6 weeks |
| Pourazar et al. 201829 | Country: Iran; N = 30; Mean age:(years old): 11.2; Type of CP: Not defined | Xbox Kinect; EG: VR and Home Physical Activity Program; CG: Physical Activity Program | 25 min | 3× week | 4 weeks |
| Piovesana e al 201769† | Country: Australia; N = 102†; Mean age:(years old): 11.7†; Type of CP: Hemiplegia † | (MiTii)†; EG: VR; CG: Waitlist† | 20-30 min† | 6× week† | 20 weeks† |
| Mitchell et al. 2016 57†† | Country: Australia; N = 101††; Mean age:(years old): 13 ††; Type of CP: Hemiplegia †† | (MiTii)††; EG: VR; CG: Waitlist control group†† | 30 min†† | 6× week†† | 20 weeks†† |
| Author/ Year | Participants characteristics | Intervention | Duration of Session | Frequency | Duration of Intervention |
|---|---|---|---|---|---|
| James et al. 201552††† | Country: Australia; N = 102†††; Mean age:(years old): 11.4 †††; Type of CP: Hemiplegia ††† | (MiTii)†††; EG: VR; CG: Waitlist ††† | 30 min††† | 6× week††† | 20 weeks ††† |
| Atasavun et al. 201663 | Country: Turkey; N = 28; Mean age:(years old): 9.6; Type of CP: Hemiplegic | Wii Sports Resort™; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 30-45 min 45 min | 2-4× week | 12 weeks |
| Urgen et al. 201658 | Country: Turkey; N = 33; Mean age:(years old): 11.2; Type of CP: Spastic hemiplegic | Wii™ Fit; EG: VR; CG: Rehabilitation Program | 45 min | 2× week | 9 weeks |
| Tarakci et al. 2016 62 | Country: Turkey; N = 38; Mean age:(years old): 10.5; Type of CP: Hemiplegic, diplegic, dyskinetic and without description | Wii™ balance board e Wii™ Fit; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 50 min | 2× week | 12 weeks |
| Ren et al. 201654 | Country: China; N = 35; Mean age:(years old): 4.6; Type of CP: Spastic diplegia | Not specified; EG: VR and Occupational Rehabilitation; CG: Conventional and Occupational Rehabilitation | - | - | 3 months |
| Preston et al 201653 | Country: UK; N = 15; Mean age:(years old): 9.2; Type of CP: Not defined | Custom Computer for Rehabilitation; EG: VR/ Botulinum Toxin; CG: Usual Rehabilitation/ Botulinum Toxin | 30 min | Average 14 sessions total | 12 weeks |
| Cho et al 201655 | Country: Republic of Korea; N = 18; Mean age:(years old): 9.8; Type of CP: Spastic | Treadmill Training with Virtual Reality (Nintendo Wii); EG: VR and Conventional Rehabilitation; CG: Treadmill Training and Conventional Rehabilitation | 30 min | 3× week | 8 weeks |
| Author/ Year | Participants characteristics | Intervention | Duration of Session | Frequency | Duration of Intervention |
|---|---|---|---|---|---|
| Bedair et al 201647 | Country: Egypt; N = 40; Mean age:(years old): 7.15; Type of CP: Spastic hemiplegia | Xbox Kinect; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 30-60 min 60 min | 3× week | 4 months |
| Acar et al 201646 | Country: Turkey; N = 30; Mean age:(years old): 9.6; Type of CP: Hemiparetic | Nintendo Wii; EG: VR and Bobath; CG: Bobath | 60 min 45 min | 2× week | 6 weeks |
| Zoccolillo et al 201520 | Country: Italy; N = 22; Mean age:(years old): 6.8; Type of CP: Not defined | Xbox Kinect; EG: VR; CG: Conventional Rehabilitation (Bobath Principles) | 30 min | 2× week | 8 weeks |
| AlSaif and Alsenany 201551 | Country: Saudi Arabia; N = 40; Mean age:(years old): -; Type of CP: Spastic diplegia | Wii™ Fit; EG: VR; CG: No Intervention | 20 min | 7× week | 12 weeks |
| Arenas and Bravos 2014 60 | Country: Chile; N = 32; Mean age:(years old): -; Type of CP: Hemiparetic | Wii™ Fit; EG: VR; CG: No Treatment CG: Conventional Rehabilitation | 30 min | - | 13 sessions |
| Chiu et al 201442 | Country: Taiwan; N = 62; Mean age:(years old): 9.5; Type of CP: Spastic hemiplegia | Wii Sports Resort™; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 40 min | 3× week | 6 weeks |
| Klobucka et al. 201366 | Country: Serbia; N = 42; Mean age:(years old): 8.1; Type of CP: Not defined | Virtual reality environment; EG: VR and Marching Training on lokomat; CG: Marching Training on lokomat | - | 3-5× week | 20 sessions (4-6 weeks) |
| Author/ Year | Participants characteristics | Intervention | Duration of Session | Frequency | Duration of Intervention |
|---|---|---|---|---|---|
| Chen et al. 201319 | Country: Taiwan; N = 27; Mean age:(years old): 8,6; Type of CP: Spastic hemiplegia and spastic diplegia | Eloton SimCycle Virtual Cycling System; EG: VR and ("Strengthening Program" Physical Therapy); CG: General Physical Activity | 40 min | 3× week | 12 weeks |
| Chen et al. 201259 | Country: Taiwan; N = 27; Mean age:(years old): 8.6; Type of CP: Spastic hemiplegia and spastic diplegia | Eloton SimCycle Virtual Cycling System; EG: VR and ("Strengthening Program" Physical Therapy); CG: General Physical Activity | 40 min | 3× week | 12 weeks |
| Sharan et al. 201245 | Country: India; N = 16; Mean age:(years old): 9.6; Type of CP: Not defined | Wii™ balance board e Wii™ Fit; EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 30 min | 3× week | 3 weeks |
| Salem et al. 201256 | Country: USA; N = 40; Mean age:(years old): 4.2; Type of CP: Neuropsychomotor Development Delay | Nintendo Wii Sports™ and Nintendo Wii Fit™; EG: VR; CG: Conventional Rehabilitation | 30 min | 2× week | 10 weeks |
| Ritterband-Rosenbaum et al. 201268 | Country: Denmark; N = 40; Mean age:(years old): 11.5; Type of CP: Spastics hemiplegia | (MiTii); EG: VR; CG: No Intervention | 30 min | 7× week | 20 weeks |
| Rostami et al. 201244 | Country: Iran; N = 32; Mean age:(years old): 8.1; Type of CP: Hemiparetic | E-Link; EG1: VR; EG2: VR and mCIMT; CG1: mCIMT; CG2: Conventional Rehabilitation | 90 min 90 min 90 min 30 min | 3× week 2× week | 4 weeks of summer |
| Ramstrand and Lygnegård. 201261 | Country: Sweden; N = 18; Mean age:(years old): 13.2; Type of CP: Hemiplegia and diplegia | Wii™ balance board e Wii™ Fit; EG: VR; CG: No intervention | Minimum of 30 min | 5× week | 5 weeks |
| Author/ Year | Participants characteristics | Intervention | Duration of Session | Frequency | Duration of Intervention |
|---|---|---|---|---|---|
| Wade et al. 201265 | Country: UK; N = 19; Mean age:(years old): 9.1; Type of CP: Not defined | Computer and equipment with seat and platform; GE: VR; GC: No intervention | - | (3-5× week) | 3 months |
| Jannink et al. 200849 | Country: Netherlands; N = 10; Mean age:(years old): 12.1; Type of CP: Tetraplegia, diplegia and hemiplegia | Eye Toy-Play system (Play station 2); EG: VR and Conventional Rehabilitation; CG: Conventional Rehabilitation | 30 min | 2× week | 6 weeks |
| Reid and Campbell. 200643 | Country: Canada; N = 31; Mean age:(years old): 9.5; Type of CP: Not defined | RV IREX System; EG: VR and physiotherapy/ occupational; CG: Physiotherapy/ occupational | 90 minutes | 1× week | 8 weeks |
| Harris and Reid 200570 | Country: Canada; N = 16; Mean age:(years old): 10.1; Type of CP: Spastics, quadriplegia, diplegia; hemiplegia and athetoid | RV IREX System; EG: VR and Physical / Occupational Therapy; CG: Physical / Occupational Therapy | 60 min | - | 8 sessions |
| Akhutina et al. 200367 | Country: Russia; N = 1st (15) 2nd (51); Mean age(years old): 10.2; Type of CP: 1st (Cerebral palsy) 2nd (Diplegics, hemiparesis, anatomical statical and hyperkinetics). | VRT Super Scape 3D; EG: VR and Non-Specific Rehabilitation; CG: Non-Specific Rehabilitation Training | 30–60 min | 6-8× per month | 4 weeks |
Abbreviations: CP, Cerebral palsy; EG, Experimental group; CG Control group; mCIMT, Modified Induced Containment Therapy; MiTii, Move It to Improve It; IREX VR, System Interactive Rehabilitation and Exercise Systems/ Mandala® Gesture Xtreme; E-Link, E-Link evaluation and exercise system.
† Outcomes related to Piovesana et al study.69
†† Outcomes related to Mitchel et al study.57
††† Outcome related to James et al study.52
In general, the trials used VR for training upper limb function,26,30,31,42–54 lower limbs,19,26,27,50,51,54–59 postural control and balance,24,27,28,45,50,55,58,60–65 global motor function,19,24,27,30,46,47,51,54–59,62,63,66 spatial functions, cognition, and perceptions,23,29,50,52,67–69 and motivational influence.43 The use of VR ranged from custom technological resources27,31,44,52,53,57,65,67–69 to consoles commercially available as Nintendo Wii.42,45,46,48,50,51,55,56,58,60–64 Session duration ranged from 2051,27,29,49,52,53,55,56,60,61,64,67,68 to more than 60 minutes26,28,31,43,44,46–48,67,70with some trials with unspecified time.45,65 The frequency of treatment sessions ranged from two20,23,24,30,43,46,49,53,56,58,62,67 to more than three times per week,26–28,31,50–52,61,63,65,66,68 with two trials not reporting the frequency.60,70 In general, interventions ranged from four to twenty weeks in duration. The use of VR combined with conventional versus conventional rehabilitation was analyzed in 25 trials,19,20,23,24,26,27,29,30,42–50,54,55,59,62,63,66,67,70 VR versus conventional rehabilitation in eight trials,25,28,31,53,56,58,60,64 and VR versus waiting list in five trials.51,52,61,65,68
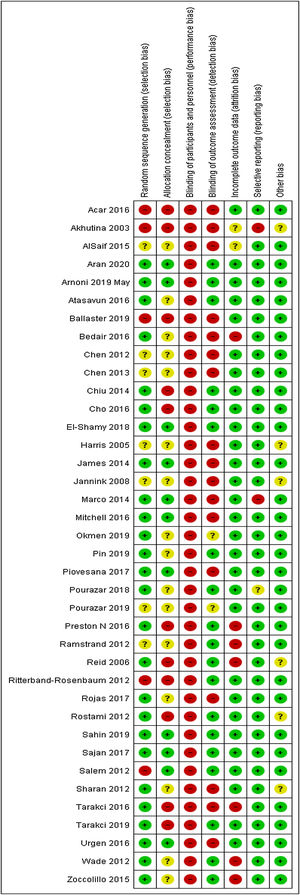
Risk of biasRisk of bias of the 38 eligible trials are described in Fig. 2. A total of 26 trials performed adequate randomization 23,24,26,27,29–31,42–45,47,48,50,52,53,55,57,58,60,62–65,69,71 Only 11 trials performed allocation concealment.23,24,30,48,50,52,56–58,60,69 All trials were classified as having a high risk of bias for blinding of participants and therapists. A total of 29 trials provided complete information on the outcomes analyzed.19,23,24,26–31,42,44–46,48–50,52,55–60,63,64,68–70 A total of 35 trials reported all of the outcomes analyzed.19,23,24,26–28,30,31,42–53,55–59,61–65,68–71
Primary outcomesEffect of VR plus conventional rehabilitation versus conventional rehabilitationWe included 11 trials that analyzed upper limb function.20,26,30,42–48,50 There is very low quality evidence (downgraded due to risk of bias, inconsistency, and imprecision) that combining VR to a conventional rehabilitation program results in improved function of the upper limb (large effect) at post intervention (SMD = 1.06, 95% CI 0.42, 1.69; n = 390, 11 trials, I2 = 86%) and at short-term follow-up (SMD = 3.04, 95% CI 0.43, 5.66; n = 93, 2 trials, I2 = 89%) when compared to a conventional rehabilitation program alone (Fig. 3).
Function of the lower limbs related to gait and strength were analyzed in five trials.19,26,27,50,55 There is low quality evidence (downgraded due to risk of bias and imprecision) that there is no benefit of adding VR to a conventional rehabilitation to improve gait at post intervention (SMD = -0.08, 95% CI -0.45, 0.29; n = 123, 5 trials, I2 = 6%).50,55,62 There is low quality evidence (downgraded due to risk of bias and inaccuracy) that adding VR to conventional rehabilitation leads to better improvement of lower limb strength (large effect) at post intervention (SMD = 0.66, 95% CI 0.01, 1.32; n = 45, 2 trials, I2 = 12%) (Supplemental online material).
Seven trials24,27,45,50,55,62,63 analyzed balance. There is very low quality evidence (downgraded due to risk of bias, inconsistency, and imprecision) that there is no benefit of adding VR to a conventional rehabilitation program to improve postural control and balance (SMD = 0.43, 95% CI -0.11, 0.97; n = 124, 6 trials, I2 = 53%) (Supplemental online material). One trial24 demonstrated no difference for the improvement of postural stability (p > 0.05).
Effect of VR versus conventional rehabilitationWe included six trials that analyzed upper limb function.25,31,44,49,53,56 There is very low quality evidence (downgraded due to risk of bias, inconsistency, and imprecision) that there is no benefit between the use of VR versus conventional rehabilitation to improve upper limb function at post intervention (SMD = 0.48, 95% CI -0.47, 1.43; n = 148, 4 trials, I2 = 79%) and at short term follow-up (SMD = 3.95, 95% CI -4.86, 12.75; n = 24, 2 trials, I2 = 93%) (Supplemental online material).
Two trials56,58 analyzed gait-related lower limb function. There is low quality evidence (downgraded due to risk of bias and imprecision) that there is no benefit between the use of VR versus conventional rehabilitation to improve gait speed at post intervention (MD 0.33, 95% CI -0.09, 0.75; n = 110, 2 trials, I2 = 0%) and functional strength post intervention (MD -0.30, 95% CI -1.49, 0.89; n = 80, 1 trial, I2 = 0%) (Supplemental online material).
Four trials analyzed balance.56,58,60,64 There is a very low quality evidence (downgraded due to risk of bias, inconsistency, and imprecision) that there is a large effect of VR versus conventional rehabilitation to improve postural control and balance at post intervention (SMD = 1.43, 95% CI 0.61, 2.24; n = 142, 3 trials, I2 = 74%) and also low quality evidence (downgraded due to risk of bias and imprecision), that there is no benefit between the use of VR versus conventional rehabilitation to improve postural control and balance at short term follow-up (SMD = 0.41, 95% CI -0.29, 1.11; n = 32, 1 trial, I2 = 0%) (Supplemental online material). One trial28 demonstrated the superiority of VR to improve postural stability (p < 0.05).
Effect of VR versus waiting list/no treatmentTwo trials51,52 analyzed upper limb function. There is very low quality evidence (downgraded due to risk of bias, inconsistency, and imprecision) that there is no benefit between the use of VR versus no treatment to improve upper limb function at post intervention (SMD = 0.64, 95% CI -0.40, 1.67; n = 115, 2 trials, I2 = 84%) (Supplemental online material).
Two trials51,57 analyzed gait-related lower limb function. There is low quality evidence (downgraded due to risk of bias and imprecision) that there is a moderate effect of using VR versus no treatment to improve gait at post intervention (SMD 0.67, 95% CI 0.13, 1.21; n = 129, 2 trials, I2 = 49%) and a large effect to improve functional strength at post intervention (MD 2.60, 95% CI 1.03, 4.17; n = 89, 1 trial, I2 = 0%) (Supplemental online material).
The summary of findings and the overall quality of evidence for the primary outcomes are described in Table 2.
Quality of evidence assessment (GRADE).
| Quality assessment (GRADE) | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Number of participants | Effect | |
| VRCR | CR | Effect Size (95% CI) | |||||||
| Upper limb function - post intervention | |||||||||
| 11 RCTs | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 196 | 194 | SMD 1.06 (0.42, 1.69) |
| Upper limb function - Short term | |||||||||
| 2 RCTs | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 42 | 51 | SMD 3.04 (0.43, 5.66) |
| Lower limb function (Gait) - post intervention | |||||||||
| 5 RCTs | Seriousa | Not Serious | Not Serious | Seriousc | Undetected | ≍≍⨁⨁ Low | 62 | 61 | SMD -0.08 (-0.45, 0.29) |
| Lower limb function (Gait) - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Lower limb function (Strength) - post intervention | |||||||||
| 2 RCTs | Seriousa | Not Serious | Not Serious | Seriousc | Not Serious | ≍≍⨁⨁ Low | 22 | 23 | SMD 0.66 (0.01, 1.32) |
| Lower limb function (Strength) - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Postural control and Balance - post intervention | |||||||||
| 6 RCTs | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 62 | 62 | SMD 0.43 (-0.11, 0.97) |
| Postural control and Balance - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Quality assessment (GRADE) | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Number of studies | Effect | |
| VR | CR | Effect Size (95% CI) | |||||||
| Upper limb function - post intervention | |||||||||
| 4 RCTs | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 70 | 78 | SMD 0.48 (-0.47, 1.43) |
| Upper limb function - Short term | |||||||||
| 1 RCT | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 8 | 16 | SMD 3.95 (-4.86, 12.75) |
| Lower limb function (Gait) - post intervention | |||||||||
| 2 RCTs | Seriousa | Not Serious | Not Serious | Seriousc | Undetected | ≍≍⨁⨁ Low | 55 | 55 | MD 0.33 (-0.09, 0.75) |
| Lower limb function (Gait) - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Lower limb function (Strength) - post intervention | |||||||||
| 1 RCT | Seriousa | Not Serious | Not Serious | Seriousc | Undetected | ≍≍⨁⨁ Low | 40 | 40 | MD -0.30 (-1.49, 0.89) |
| Lower limb function (Strength) - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Postural control and Balance - post intervention | |||||||||
| 3 RCTs | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 56 | 56 | SMD 1.43 (0.6, 2.24) |
| Postural control and Balance - Short term | |||||||||
| 1 RCT | Seriousa | Not Serious | Not Serious | Seriousc | Undetected | ≍≍⨁⨁ Low | 16 | 16 | MD 0.48 (-0.31, 1.27) |
| Quality assessment (GRADE) | Summary of findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Number of studies | Effect | |
| VR | Control | Effect Size (95% CI) | |||||||
| Upper limb function - post intervention | |||||||||
| 2 RCTs | Seriousa | Seriousb | Not Serious | Seriousc | Undetected | ≍≍≍⨁ Very low | 58 | 57 | SMD 0.64 (-0.4, 1.67) |
| Upper limb function - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Lower limb function (Gait) - post intervention | |||||||||
| 2 RCTs | Seriousa | Not Serious | Not Serious | Seriousc | Undetected | ≍≍⨁⨁ Low | 66 | 63 | SMD 0.67 (0.13, 1.21) |
| Lower limb function (Gait) - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Lower limb function (Strength) - post intervention | |||||||||
| 1 RCT | Seriousa | Not Serious | Not Serious | Seriousc | ≍≍⨁⨁ Low | 46 | 43 | MD 2.60 (1.03, 4.17) | |
| Lower limb function (Strength) - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Postural control and Balance - post intervention | |||||||||
| - | - | - | - | - | - | - | - | - | - |
| Postural control and Balance - Short term | |||||||||
| - | - | - | - | - | - | - | - | - | - |
Abbreviations: GRADE, Grading of Recommendations Assessment Development and Evaluation; RCTs, randomized controlled trials; VRCR, virtual reality plus conventional rehabilitation; CR, conventional rehabilitation; MD, mean difference; SMD, standardised mean differences; CI, confidence interval; VR, virtual reality.
A total of nine trials analyzed global motor function,19,24,27,30,46,47,55,62,63 three trials spatial function, perception, and cognition29,50,67 for the effect of VR combined to a conventional rehabilitation versus conventional rehabilitation alone. Two trials analyzed global motor function56,58 for the effect of VR versus conventional rehabilitation. Two trials analyzed global motor function49,58 and two trials spatial and executive functions68,69 for the effect of VR versus waiting list or no treatment. Most of the comparisons demonstrated low quality of evidence. The benefits of VR added to a conventional rehabilitation program or used alone is unclear and may result in a slight improvement in spatial perception and executive functions. The evidence suggests that adding VR to conventional rehabilitation or used alone results in little to no difference in global motor function. Further details can be found in the Supplemental online material.
Sensitivity analysis and subgroupsThe majority of the subgroup and sensitivity analyzes did not demonstrate significant differences.
Publication BiasWe did not assess publication bias because few studies were included in the review.
DiscussionVR has been used in clinical rehabilitation for the past two decades. We found very low-quality evidence that there is a moderate effect of adding VR to conventional rehabilitation compared to conventional rehabilitation alone for improvement in upper limb function at post intervention and short term follow-up. Further, there is very low-quality evidence that there is a moderate effect of using VR compared to conventional rehabilitation for improvement in postural control and balance at post intervention. Also, there is low quality of evidence of small to large effect of VR compared to no treatment for improvement in gait and strength of the lower limb at intermediate term. Although there has been an increase in the number of publications investigating the effects of VR alone or associated with other interventions. The effect of the intervention remains unclear for most of the comparisons, outcomes and follow-up. At present, we have limited confidence in the effect estimates and the results should be interpreted with cautions.
One of the main hypotheses for the benefit of VR in rehabilitation is related to the simultaneous stimulation of cognitive and motor processes.72,73 VR enables a rewarding and challenging virtual environment in a recreational and fun context to engage in new experiences.72,74 The use of VR also enables encouragement and motivation to solve problems in different contexts. It also provides social participation, flexibility of use, feedback, and accomplishment of repetitive tasks at different difficulty levels, promoting neuroplasticity.75,76,77 The results of the review also indicated greater effect of VR compared to other treatments or no treatment for improvement of upper limb function, postural control, gait, and strength of lower limbs. Most participants were children from rehabilitation centers, so the clinician should consider patient preference and the present evidence to suggest the use or not of VR as an adjuvant to rehabilitation. It is essential at this stage that evidence-based practice in pediatric physiotherapy is considered to provide the best health care for children and families.78
We analyzed the use of VR in six intervention approaches for children with CP. At this time, the evidence related to the use of VR is based on 1233 patients from 19 different countries. The risk of bias varied substantially between the trials included in the systematic review. The reporting of the application of VR and classification of CP type was not clear in some studies, and only few trials reported on potential risks and adverse events.19,20,50,52,53,56,60,64 Most of the evidence related to VR rehabilitation has been obtained either immediately post intervention or at short-term follow-up,42,44,64 with no information at intermediate and long-term follow-up.
This is the first systematic review to our knowledge to analyze risk of bias in VR trials for individuals with CP and to use the GRADE approach to determine the quality of evidence. Previous reviews included studies of varied designs,79,80 and used diverse instruments to assess methodological quality and bias.22,77,79,81 In the present review we used the Cochrane risk of bias tool35 and reported the review according to PRISMA guidelines.82 We conducted an extensive search in the main databases, but trials stored in the gray literature were not included, therefore, the likelihood of publication bias cannot be ruled out.83,84 Some trials showed little compliance for concealed allocation, blinding of participants, and therapists which may have influenced the effect estimates.85,86
Despite the recent growth in number of trials, heterogeneity in the design of those trials and intervention comparisons, make definitive conclusions difficult. Strategy for conducting multicenter studies could be an approach to obtain larger sample sizes. Better reporting of the interventions, and the use of the TIDieR checklist87,88 is also needed to add transparency and reproducibility of interventions. It would be important to also develop a minimum set of outcomes for conducting trials in this population.89, 90 This strategy would reduce variability of measurement instruments, outcomes, and would help standardization of the frequency of interventions and follow-up periods.
ConclusionsOur findings suggest that VR demonstrates some short-term benefits to improve upper and lower limb function and postural control and balance in individuals with CP. However, the quality of evidence ranged from very low to low, with very limited confidence in the effect estimate.
FundingNone.
Conflict of InterestThe authors of this review declare no conflict of interest.
We thank Leonardo O. P. Costa for critical revision of the manuscript and assistance in data analysis.