There is no systematic review assessing the effectiveness of interferential current (IC) in patients with low back pain.
ObjectiveTo investigate the effectiveness of IC in patients with chronic non-specific low back pain.
MethodsThe databases PUBMED, EMBASE, PEDro, Cochrane Library, CINAHL, and SCIELO were searched. Randomized controlled trials reporting pain intensity and disability in patients with chronic non-specific low back pain, in which IC was applied were included. Methodological quality was assessed using the PEDro scale. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to evaluate the quality of evidence.
ResultsThirteen RCTs were considered eligible for this systematic review (pooled n = 1367). Main results showed moderate-quality evidence and moderate effect sizes that IC probably reduces pain intensity and disability compared to placebo immediately post-treatment (Pain: MD = -1.57 points; 95% CI -2.17, -0.98; Disability: MD = -1.51 points; 95% CI -2.57, -0.46), but not at intermediate-term follow-up. Low-quality evidence with small effect size showed that IC may reduce pain intensity (SMD = -0.32; 95% CI -0.61, -0.03, p = 0.03) compared to TENS immediately post-treatment, but not for disability. There is very low-quality evidence that IC combined with other interventions (massage or exercises) may not further reduce pain intensity and disability compared to the other interventions provided in isolation immediately post-treatment.
ConclusionModerate-quality evidence shows that IC is probably better than placebo for reducing pain intensity and disability immediately post-treatment in patients with chronic non-specific low back pain.
Low back pain is the world's largest health problem in terms of years lived with disability.1,2 The prevalence increases from 30 to 60 years old, being more prevalent in female. Low back pain can reach up to 65% of the population per year, and 84% of people will experience at least one episode of low back pain in their lifetime.3,4 Low back pain generates high costs with absenteeism5 and disability6 and is considered the health condition that generates the highest medical cost in the world.5,6
Analgesic electrical currents are widely used by physical therapists in their clinical practice.7 Interferential current (IC) is the most commonly used analgesic current in physical therapy services in Canada, Australia, and England.8 IC is a medium-frequency alternating current, with frequencies ranging from 1 to 10 kHz and with a modulated amplitude.9 Because it is a medium-frequency current, the IC has, in theory, the ability to reduce the skin's impedance, thus increasing penetration into the tissues and generating less discomfort to the patient,9,10 which in turn may be associated with greater effectiveness in pain relief.11,12
Some studies have showed that IC seems better than placebo for reducing pain intensity13 in patients with chronic low back pain. Systematic reviews have showed that IC seems to be more effective than control or placebo treatment.8,15 However, these findings were in patients with musculoskeletal pain.8,15
Only one systematic review,16 published in 2008, evaluated the effectiveness of electrophysical interventions, including IC, in patients with low back pain. Although many studies have evaluated analgesic currents in patients with non-specific chronic low back pain, most of these have evaluated low-frequency pulsed currents, often referred to as transcutaneous electrical nerve stimulation (TENS).17–19 To date, there is no specific systematic review assessing the effectiveness of IC in patients with chronic non-specific low back pain.
Therefore, a review summarizing the results of IC treatment (following the recommendations of the Cochrane collaboration20) is necessary to verify the possible effects of this intervention. Thus, the objective of this systematic review is to investigate the effectiveness of IC in patients with chronic non-specific low back pain.
MethodsThis systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (PRISMA Statement),21 and it was prospectively registered in the International Prospective Register of Systematic Reviews - PROSPERO (registration number CRD42013005083).
Study designs and participantsOnly randomized controlled trials evaluating the effectiveness of IC in patients with chronic non-specific low back pain were included. Participants should present with an episode of chronic pain (i.e., symptoms lasting 12 weeks or more).22 Studies that did not report the duration of symptoms were not included in the review.
Types of interventionsThe comparisons of interest were: IC versus no intervention; IC versus placebo; IC versus other interventions (e.g., TENS and manual therapy); and additional effect of IC when combined with other interventions.
OutcomesThe outcomes of interest were pain intensity and disability as assessed by validated questionnaires. No other outcomes were considered for this review. According to the Core Outcome Set for patients with low back pain, the outcomes pain and disability were considered the most important for patients with low back pain.23,24
Search strategySearches were performed on PUBMED, EMBASE, PEDro, Cochrane Library, CINAHL, and SCIELO, up to January 30th 2023. The terms used were based on the strategies suggested by the Cochrane Back and Neck Group (Supplementary material – Search Strategy), and the searches were adjusted for each database. There were no restrictions regarding language and date of publication for the potentially eligible studies.
SelectionTwo independent reviewers selected the studies by reading titles, abstracts, and full texts. Any disagreement between the reviewers were resolved by consensus, and if necessary, a third reviewer was asked to decide on the inclusion of the study. The process of study selection for this systematic review involved: (1) exclusion of duplicate trials; (2) analysis and selection by title and abstract; and (3) analysis and selection by full text.
Data extractionData related to the number and characteristics of participants (age and duration of symptoms) were extracted; as well as description of the intervention and the different comparisons; tools used to assess the outcomes; time points and results of the studies. When necessary, the authors of the included studies were contacted to request additional information on the data. Data presented in graphs were extracted using WebPlotDigitizer v4.0.25
Quality assessmentTo assess the methodological quality and statistical reporting of the included trials, the PEDro scale was used.26,27 The scores were extracted from the PEDro database itself, and studies that were not indexed in the PEDro database were rated by two trained independent reviewers. The PEDro scale evaluates the methodological quality and statistical reporting of randomized controlled trials. This scale has 11 items: eight items related to methodological quality (i.e., random allocation, concealed allocation, baseline comparability, blinded subjects, blinded therapists, blinded assessors, adequate follow-up, and intention-to-treat analysis) and two items related to statistical reporting (between-group comparisons and point estimates and variability).26,27 The first item (eligibility criteria) is related to external validity; therefore, it is not considered in the total score that ranges from 0 to 10. It has been suggested that scores of: < 4 are considered ‘poor’, 4 to 5 are considered ‘fair’, 6 to 8 are considered ‘good’, and 9 to 10 are considered ‘excellent.’28
Analysis and synthesisThe mean difference between groups and the respective 95% confidence intervals were calculated and used to quantify the treatment effect for continuous outcomes. In meta-analyses, for studies using the same scale, the results were expressed as mean difference (MD) and 95% confidence intervals. Otherwise, the treatment effects were calculated through standardized mean differences (SMD) and 95% confidence intervals. The effect size of the interventions was classified as small (MD < 10% of the scale or SMD 0.4), moderate (MD = 10% to 20-% of the scale or SMD = 0.41 to 0.7), or large (MD > 20% of the scale or SMD > 0.7).29 The treatment effect was considered clinically significant when there was a difference between groups of at least 20% in the outcome analyzed.30 When possible, meta-analyses were based on time of follow-up, that is, immediately post-treatment: up to one day; short-term follow-up: more than one day up to 3 months; intermediate-term follow-up: more than 3 months up to 1 year; long-term follow-up: more than 1 year) or based on treatment duration (number of sessions). Regardless of the heterogeneity between the studies included in the meta-analyses, only the random effects model was adopted. For studies in which standard deviations (SD) were not presented, the largest SDs from a difference study in the same meta-analysis was imputed, following the recommendation of the Guideline for Systematic Reviews from the Cochrane Back and Neck Group.31 Three-arm trials were pooled using the method “Splitting shared group”.32,33
Quality of evidenceThe overall quality of evidence was assessed according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE).34 GRADE approach consider five domains: study design and limitations, inconsistency (I² value is greater than 50%), indirectness (population, prognostic factors, and/or outcomes do not fully represent the review question), imprecision (few participants, and thus have wide confidence intervals) and publication bias; single randomized trials were considered inconsistent and imprecise (that is, sparse data) and provided “very low quality” evidence.
The quality of the evidence was classified as: high quality of evidence - consistent results within at least 75% of the clinical trials of good methodological quality, presenting consistent, direct, and accurate data and with no suspicious or known publication bias, and further research is unlikely to alter the estimate or our confidence in the results; moderate quality of evidence - one item is not met (good methodological quality, consistent, direct, and accurate data and publication bias); low quality of evidence - two of the domains are not met, the true effect may be substantially different from the estimate of the effect; or very low quality of evidence - three domains are not met and the results are highly uncertain, the true effect is likely to be different from the estimate of effect.35–37
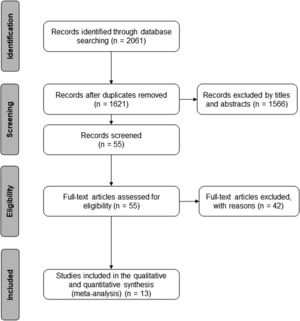
ResultsThe searches identified a total of 2061 trials. After applying the inclusion criteria, 13 randomized controlled trials were considered eligible and were included in the review (Fig. 1). The sample sizes ranged from 2838 to 28039 patients, totaling 1367 patients in the review. The characteristics of the selected trials are presented in Table 1. The trials were conducted in four different countries: eight7,38–44 in Brazil, three14,45,46 in Spain, one47 in Poland, and one48 in Nigeria. All included trials evaluated pain intensity as an outcome measure.7,14,38,40–43,45–49 Six trials7,14,38,42,45,47 rated pain using the 0–10 or 0–100 Visual Analogue Scale (VAS) and seven trials40,41,43,44,46,48,49 rated pain using the 0–10 Numeric Rating Scale (NRS). Disability outcome was evaluated in eight trials.7,14,38,40–42,45,48 Seven trials7,14,38,40–42,48 used the 0–24 Roland Morris Disability Questionnaire (RMDQ) and one trial45 used the 0–100 Oswestry Disability Index (ODI). Seven trials had more than two groups for comparisons.7,41–44,48,49
Data extraction.
| Author (Year) | Participants | Type of intervention | Results |
|---|---|---|---|
| Albornoz-Cabello et al. (2017)43 | 64 individuals with non-specific chronic low back pain, 44 women and 20 men with a mean age of 51 years. | IC Group (n = 44): Carrier frequency = 4 kHz, AMF = 65 Hz, sweep frequency = 95 Hz, 1:1 swing pattern, quadripolar technique, intensity based on tolerance (pins-and-needles) without muscle contraction, 25-minute application, five times per week, totaling 10 sessions.Control group (n = 20): soft tissue massage and mobilization, 25-minutes applications, five times a week, totaling 10 sessions. | Two-week follow-up (10 sessions):IC versus MassagePain (0–100) MD = 11.34 (95% CI 1.77, 20.91)Disability (0–100) MD = 13.38 (95% CI 4.97, 21.78) |
| Almeida et al. (2019)40 | 105 individuals with chronic low back pain, 67 women and 27 men with an average age of 39.5 years. | Interferential AMF 2 Hz Group (n = 35): participants received IC treatment with 2 kHz carrier frequency, 2 Hz AMF, intensity at motor level, one single 30-minute application.Interferential AMF 100 Hz Group (n = 35): participants received IC treatment with 2 kHz carrier frequency, 100 Hz AMF, intensity at sensory level, one single 30-minute application.Placebo group (n = 35): electrical stimulus off, one single 30-minute application. | One-session follow-up:IC AMF 2 Hz versus PlaceboPain (0–10) MD = 1.4 (95% CI 0.5, 2.3)Disability (0–24) MD= 2.9 (95% CI 1.0, 4.8)IC AMF 100 Hz versus PlaceboPain (0–10) MD = 1.7 (95% CI 0.8, 2.6)Disability (0–24) MD= 3.4 (1.5, 5.3)IC AMF 2 Hz versus IC AMF 100 HzPain (0–10) MD = −0.3 (−1.2, 0.5)Disability (0–24) MD = 0.5 (−1.3, 2.3) |
| Almeida et al. (2020)41 | 175 individuals with non-specific chronic low back pain, 105 women and 70 men with an average age of 37.7 years. | IC 2 kHz/100 Hz Group (n = 35): Carrier frequency = 2 kHz, AMF = 100 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory level, one session for 30 min.IC 2 kHz/2 Hz Group (n = 35): Carrier frequency = 2 kHz, AMF = 2 Hz, sweep frequency = 0 Hz, quadripolar technique, i = motor level, one session for 30 min.IC 4 kHz/100 Hz Group (n = 35): Carrier frequency = 4 kHz, AMF = 100 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory level, one session for 30 min.IC 4 kHz/2 Hz Group (n = 35): Carrier frequency = 4 kHz, AMF = 2 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory level, one session for 30 min.IC Placebo Group (n = 35): the procedures were similar, but the current amplitude/intensity was not increased. | One-session follow-up:IC 2 kHz/100 Hz versus IC 2 kHz/2 HzPain (0–10) MD = 0.9 (95% CI 0.1, 1.7)IC 2 kHz/100 Hz versus IC 4 kHz/100 HzPain (0–10) MD = 1.1 (95% CI 0.3, 1.8)IC 2 kHz/100 Hz versus IC 4 kHz/2 HzPain (0–10) MD = 0.7 (95% CI 0.0, 1.5)IC 2 kHz/100 Hz versus IC PlaceboPain (0–10) MD = −0.6 (95% CI −1.6, 0.4)IC 2 kHz/2 Hz versus 4 kHz/100 HzPain (0–10) MD = 0.1 (95% CI −0.5, 0.9)IC 2 kHz/2 Hz versus 4 kHz/2 HzPain (0–10) MD = −0.1 (95 % CI −0.9, 0.6)IC 2 kHz/2 Hz versus IC PlaceboPain (0–10) MD = −1.6 (95% CI −2.5, −0.7)IC 4 kHz/100 Hz versus IC 4 kHz/2 HzPain (0–10) MD = −0.3 (95% CI −1.0, 0.4)IC 4 kHz/100 Hz versus IC PlaceboPain (0–10) MD = −2.1 (95% CI −2.9, −1.3)IC 4kHz/2 Hz versus IC PlaceboPain(0–10) MD = −1.7 (95 % CI −2.6, −0.8) |
| Almeida et al. (2022)42 | 63 individuals with non-specific chronic low back pain, of both sexes, 38 women and 25 men, with a mean age of 31,36 years. | IC 4 kHz/100 Hz Group (n = 21): Carrier frequency = 4 kHz, AMF = 100 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory intensity, one session for 30 min.IC 4 kHz/ 2 Hz Group (n = 21): Carrier frequency = 4 kHz, AMF = 2 Hz, sweep frequency = 0 Hz, quadripolar technique, i = motor intensity, one session for 30 min.IC Placebo Group (n = 21): submitted to the intervention, but in this group, the equipment was turned off. | One session lasting 30 minIC 4 kHz/100 Hz versus IC 4 kHz/ 2 HzPain (0–10) MD = −0.4 (95% CI −1.4, 0.6)IC 4 kHz/100 Hz versus Placebo GroupPain (0–10) MD = −1.1 MD (95% CI −2.1, −0.1)IC 4 kHz/ 2 Hz versus Placebo GroupPain (0–10) MD = −0.7 MD (95% CI −1.7, 0.2) |
| Correa et al. (2016)39 | 150 individuals with non-specific chronic low back pain, of both sexes, 115 women and 35 men, with a mean age of 51.16 years. | IC 1 kHz Group (n = 50): Carrier frequency = 1 kHz, AMF = 100 Hz, sweep frequency, 1:1 swing pattern, bipolar technique with two channels, i = strong but comfortable tingling sensation, three times a week for four weeks.IC 4 kHz Group (n = 50): Carrier frequency = 4 kHz, AMF = 100 Hz, sweep frequency = 50 Hz, 1:1 swing pattern, bipolar technique with two channels, i = strong but comfortable tingling sensation, three times a week for four weeks.Placebo IC Group (n = 50): received 30-minute placebo treatment three times a week for four weeks. | IC 1 kHz versus PlaceboFour-week follow-up (12 sessions):Pain (0–10) MD = −1.0 (95% CI −1.9, −0.1)Disability (0–24) MD = 0.8 (95% CI −1.5, 3.1)Four-month follow-up:Pain (0–10) MD = 0.4 (95% CI 1.0, 1.1)Disability (0–24) MD = 0.3 (95% CI 2.0, 2.6)IC 4 kHz versus PlaceboFour-week follow-up (12 sessions):Pain (0–10) MD = −0.9 (95% CI −1.8, 0.0)Disability (0–24) MD = 2.1 (95% CI 0.2, 4.5)Four-month follow-up:Pain (0–10) MD = 0.2 (95% CI 0.9, 1.2)Disability (0–24) MD = 0.2 (95% CI 2.1, 2.5) |
| Dias et al. (2021)37 | 280 individuals with non-specific chronic low back pain, 179 female and 101 men, with a mean age of 39.6 years. | GI2kHz/100 Hz. IC (n = 35): carrier frequency = 2 kHz, AMF = 100 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory level, a single 30-minute session.GI2kHz/2 Hz. IC (n = 35): carrier frequency = 2 kHz, AMF = 2 Hz, sweep frequency = 0 Hz, quadripolar technique, i = motor level, a single 30-minute sessionGI4kHz/100 Hz. IC (n = 35): carrier frequency = 4 kHz, AMF = 100 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory level, a single 30-minute sessionGI4kHz/2 Hz. IC (n = 35): carrier frequency = 4 kHz, AMF = 2 Hz, sweep frequency = 0 Hz, quadripolar technique, i = sensory level, a single 30-minute sessionGIP. Placebo IC (n = 35): same procedures, but zero intensity.GT100Hz. TENS (n = 35): Frequency = 100 Hz, T = 100 µs, i = sensory level, a single 30-minute sessionGT2Hz. TENS (n = 35): Frequency = 2 Hz, T = 100 µs, i = motor level, a single 30-minute sessionGTP. Placebo TENS (n = 35): same procedures, but zero intensity | One-session follow-up:GI2kHz/100 Hz versus GI2kHz/2 HzPain (0–10) MD = 0.94 (95% CI 0.02, 1.85)GI2kHz/100 Hz versus GI4kHz/100 HzPain (0–10) MD = 1.11 (95% CI 0.19, 2.03)GI2kHz/100 Hz versus GI4kHz/2 HzPain (0–10) MD = 0.78 (95% CI −0.13, 1.70)GI2kHz/100 Hz versus GIPPain (0–10) MD = −0.01 (95% CI −0.9, 0.9)GI2kHz/100 Hz versus GT100HzPain (0–10) MD = 0.34 (95% CI −0.57, 1.25)GI2kHz/100 Hz versus GT2HzPain (0–10) MD = 0.01 (95% CI −0.90, 0.93)GI2kHz/100 Hz versus GTPPain (0–10) MD = −2.61 (95% CI −3.53, −1.69)GI2kHz/2 Hz versus GI4kHz/100 HzPain (0–10) MD = 0.17 (95% CI −0.74, 1.08)GI2kHz/2 Hz versus GI4kHz/2 HzPain (0–10) MD = −0.15 (95% CI −1.07, 0.75)GI2kHz/2 Hz versus GIPPain (0–10) MD = 0.95 (95% CI −1.87, −0.42)GI2kHz/2 Hz versus GT100HzPain (0–10) MD = −0.60 (95 % CI −1.51, 0.31)GI2kHz/2 Hz versus GT2HzPain (0–10) MD = −0.92 (95% CI −1.84, −0.01)GI2kHz/2 Hz versus GTPPain (0–10) MD = −3.55 (95% CI −4.47, −2.64)GI4kHz/100 Hz versus GI4kHz/2 HzPain (0–10) MD = −0.32 (95% CI −1.24, 0.58)GI4kHz/100 Hz versus GIPPain (0–10) MD = −1.12 (95% CI −2.04, −0.21)GI4kHz/100 Hz versus GT100HzPain (0–10) MD = −0.77 (95% CI −1.68, −0.14)GI4kHz/100 Hz versus GT2HzPain (0–10) MD = −1.10 (95% CI −2.01, −0.18)GI4kHz/100 Hz versus GTPPain (0–10) MD = −3.72 (95% CI −4.64, −2.81)GI4kHz/2 Hz versus GIPPain (0–10) MD = −0.8 (95% CI −1.7, 0.1)GI4kHz/2 Hz versus GT100HzPain (0–10) MD = −0.44 (95% CI −1.35, 0.47)GI4kHz/2 Hz versus GT2HzPain (0–10) MD = −0.77 (95% CI −1.68, 0.14)GI4kHz/2 Hz versus GTPPain (0–10) MD = −3.40 (95% CI −4.31, −2.48)GT100Hz versus GIPPain (0–10) MD = −0.35 (95% CI −1.27, 0.55)GT2Hz versus GIPPain (0–10) MD = −0.29 (95% CI −0.94, 0.88)GT100Hz versus GT2HzPain (0–10) MD = −0.32 (95% CI −1.24, 0.58)GT100Hz versus GTPPain (0–10) MD = −2.95 (95% CI −3.87, −2.04)GT2Hz versus GTPPain (0–10) MD = −2.62 (95% CI −3.54, −1.71) |
| Dohnert et al. (2015)36 | 28 individuals with non-specific chronic low back pain, 22 women and 6 men, with a mean age of 61 years. | Intervention Group (IC) (n = 14): carrier frequency = 4 kHz, AMF = 20 Hz, sweep frequency = 10 Hz 1:1 swing pattern, i = based on patient tolerance, 30-minute sessions, twice a week for five weeks.Control Group (TENS) (n = 14): acupuncture mode, F = 20 Hz and T = 100 µs, i = according to patient tolerance, 30-minute sessions twice a week for five weeks. | Five-week follow-up (10 sessions):IC versus TENSPain (0–100) MD = 11.34 (95% CI 1.77, 20.91) / Disability (0–100) MD = 13.38 (95% CI 4.97, 21.78) |
| Espejo-Antúnez et al. (2021)44 | 49 males with non-specific chronic low back pain, and a mean age of 39 years. | IC Group (n = 25): Carrier frequency = 4 kHz, AMF = 65 Hz, sweep frequency = 95 Hz, 1:1 swing pattern, quadripolar technique, I = “pins and needles” sensation, but without visible muscle twitches, 25 min of 1 single session.Sham Group (n = 24): received the sham intervention. | One-session follow-up:IC versus ShamPain (0–10) MD = −3.56 (95% CI −4.17, 2.95) |
| Facci et al. (2011)6 | 150 individuals with non-specific chronic low back pain, 109 women and 41 men, with a mean age of 47.2 years. | Interferential Current Group (n = 50): carrier frequency = 4 kHz, AMF = 20 Hz, sweep frequency = 10 Hz, 1:1 swing pattern, quadripolar technique, i = strong but comfortable, adjusted according to each patient's sensitivity, 30-minute sessions, twice a week for two weeks.TENS group (n = 50): Frequency = 20 Hz, T = 330 ms, 2 channels, i = strong but comfortable, adjusted according to each patient's sensitivity, 30-minute sessions twice a week for two weeks.Control Group (n = 50): patients received no intervention for two weeks. | Two-week follow-up (10 sessions):IC versus no interventionPain (0–10) Estimated MD = 45 mmDisability Estimated MD = 7.7 mmIC versus TENSPain (0–10) MD = 0.22 (95% CI 0.03, 0.41)Disability MD = 1.17 (no statistical difference). The CI could not be calculated because the authors did not present standard deviations. The authors were contacted and did not respond to our emails. |
| Franco et al. (2017)38 | 148 individuals with non-specific chronic low back pain, of both sexes, 108 women and 40 men, with a mean age of 65.7 years. | Group IC plus Pilates group (n = 74): in the first two weeks participants received IC treatment (30 min), and in the following four weeks, IC sessions were immediately followed by 40 min of Pilates. Sessions were 3 times a week for 6 weeks. Carrier frequency = 4 kHz; AMF = 100 Hz; sweep frequency = 50 Hz; swing pattern 1:1 for 30 min, bipolar technique with two channels, i = strong but comfortable tingling sensation.Group Placebo IC plus Pilates (n = 74): in the first two weeks, participants received Placebo IC treatment (30 min) and in the following four weeks IC Placebo sessions were immediately followed by 40 min of Pilates; sessions were three times a week for six weeks. | IC plus Pilates versus Placebo IC plus PilatesSix-week follow-up (18 sessions):Pain (0–10) MD = 0.1 (95% CI −0.9, 1.0)Disability (0–24) MD = 0.4 (95% CI −1.3, 2.2)Six-month follow-up:Pain (0–10) MD = −0.3 (95% CI −1.2, 0.6)Disability (0–24) MD = 0.3 (95% CI −1.4, 2.1) |
| Grabianska et al. (2015)45 | 60 individuals with non-specific chronic low back pain, mean age 53.5 years. | IC group (n = 30): received treatment distributed in 10 sessions (20 min each), frequency = 90–100 Hz.TENS group (n = 30): received F = 100 Hz, T = 150 US for 20 min, once daily, totaling 10 sessions. | Two-week follow-up (10 sessions):IC versus TENSPain (0–10) MD = −0.41 (95% CI −1.04, 0.22) |
| Lara-Palomo et al. (2012)13 | 61 individuals with non-specific chronic low back pain, 41 women and 20 men, mean age 48.5 years. | Experimental Group (n = 30): superficial tissue massage plus IC. Carrier frequency = 4 kHz, AMF = 80 Hz, bipolar technique, i = 30–50 mA, always below the patient's pain threshold, for 30 min, twice a week for 10 weeks.Placebo group (n = 31): received 20 sessions (30 min each) of superficial tissue massage twice a week for 10 weeks. | Ten-week follow-up (20 sessions):IC versus Superficial Tissue MassagePain (0–10) MD = −1.06 (95% CI −1.91, −0.22)Disability (0–24) MD = −3.01 (95% CI −4.53, −1.47). |
| Tella et al. (2021)46 | 33 individuals with non-specific chronic low back pain, 23 women and 10 men, mean age 49.9 years. | IC group (n = 11): received exercises + IC: carrier frequency = 4 kHz, AMF = 150 Hz, quadripolar technique on painful area, i = 20–70 mA (strong but comfortable), for 20 min, twice a week for five weeks.TENS group (n = 11): received exercises + TENS: F = 150 Hz, four self-adhesive surface electrodes on painful area, i = 20–80 mA (strong, but comfortable) for 20 min, twice a week for five weeks.Exercise group (n = 11): received 30 min of exercises. | Five-week follow-up (10 sessions):IC versus TENSPain (0–10) MD = −0.09 (95% CI −1.16, 0.98)Disability (0–24) MD = 1.82 (95% CI −3.09, 6.73).IC versus ExercisesPain (0–10) MD = 0.09 (95% CI −1.30, 1.48)Disability (0–24) MD = 1.72 (95% CI −2.71, 6.15). |
AMF, amplitude-modulated frequency; TENS, transcutaneous electrical nerve stimulation; IC, interferential current; EG, experimental group; CG, control group; US, ultrasound; MD, mean difference; SMD, standard mean difference; CI, confidence interval; Hz, hertz; kHz, kilohertz; n, number of participants; T, pulse duration; i, intensity.
Eleven studies used a carrier frequency of 4 kHz,7,14,38,40,41,43–46,48,49,47 three used 2 kHz,42,43,49 one used 1 kHz,41 and one study did not provide the frequency used.47 The amplitude-modulated frequency (AMF) of 2 Hz was used in four studies,42–44,49 20 Hz in two,7,38 65 Hz in two,45,46 80 Hz in one,14 100 Hz in six,40–44,49 150 Hz in one,48 and 90–100 Hz in one study.47 Quadripolar technique was used in most studies with exception of one study that used bipolar technique14 and one study that did not provide any information.47 Regarding the number of sessions, a single session was used in five studies,42–44,46,49 10 sessions in five studies,7,38,45,47,48 12,41 18,40 and 2014 sessions were applied in one study each. The treatment time per session for IC application was of 20,47,48 25,45,46 or 307,14,38,40–44,49 minutes.
The methodological quality of the studies is described in Table 2. Nine studies were identified in the PEDro database.7,14,38,40,41,43,45,47,49 Methodological quality scores ranged from 4 to 9 with a mean score of 6.2 (SD = 1.5) (Table 2). The overall quality of evidence according to GRADE was rated as very low to moderate (Table 3).
Methodological quality and statistical reporting of eligible trials.
| Author (Year) | PEDro ITEMSa | PEDro score (0-10) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Albornoz-Cabello et al. (2017)43 | Y | Y | Y | N | N | N | N | Y | Y | Y | Y | 6 |
| Almeida et al. (2019)40 | Y | Y | N | Y | Y | N | N | Y | N | Y | Y | 6 |
| Almeida et al. (2020)41 | N | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Almeida et al. (2022)42 | N | Y | N | Y | Y | N | Y | Y | Y | Y | Y | 8 |
| Correa et al. (2016)39 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 9 |
| Dias et al. (2021)37 | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Dohnert et al. (2015)36 | N | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Espejo-Antúnez (2021)44 | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Facci et al. (2011)6 | Y | Y | Y | Y | N | N | N | Y | Y | Y | N | 6 |
| Franco et al. (2017)38 | Y | Y | Y | Y | N | N | N | Y | Y | Y | Y | 7 |
| Grabianska et al. (2005)45 | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4 |
| Lara-Palomo et al. (2012)13 | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 |
| Tella et al. (2021)46 | Y | Y | N | Y | Y | N | N | Y | N | Y | Y | 6 |
Y: Yes; N: No.
1: eligibility criteria and source of participants; 2: random allocation; 3: concealed allocation; 4: baseline comparability; 5: blinded participants; 6: blinded therapists; 7: blinded assessors; 8: adequate follow-up; 9: intention-to-treat analysis; 10: between group comparison; 11: point estimates and variability.
Summary of findings and quality of evidence (GRADE).
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Outcome | Timepoint | n (IC group) | n (Comparison group) | Effect (absolute risk 95 % CI) | Quality of evidence (GRADE) | Importance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interferential Current versus Placebo | ||||||||||||
| 7 | RCT | Not serious | Serious | Not serious | Not serious | Pain intensity | Immediately post-treatment | 591 | 274 | MD −1.57 (−2.17, −0.98) | ⨁⨁⨁◯a MODERATE | Critical |
| 2 | RCT | Not serious | Not serious | Not serious | Serious | Pain intensity | Intermediate-term follow-up | 174 | 124 | MD −0.02 (−0.70, 0.65) | ⨁⨁⨁◯b MODERATE | Important |
| 3 | RCT | Not serious | Not serious | Not serious | Serious | Disability | Immediately post-treatment | 244 | 159 | MD −1.51 (−2.57, −0.46) | ⨁⨁⨁◯b MODERATE | Critical |
| 2 | RCT | Not serious | Not serious | Not serious | Serious | Disability | Intermediate-term follow-up | 174 | 124 | MD −0.72 (−2.27, 0.83) | ⨁⨁⨁◯b MODERATE | Important |
| Interferential Current versus TENS | ||||||||||||
| 5 | RCT | Serious | Serious | Not serious | Not serious | Pain intensity | Immediately post-treatment | 245 | 175 | MD −0.32 (−0.61, −0.03) | ⨁⨁◯◯c LOW | Critical |
| 2 | RCT | Serious | Serious | Serious | Very Serious | Disability | Immediately post-treatment | 25 | 25 | MD −0.67 (−3.72, 2.38) | ⨁◯◯◯d VERY LOW | Critical |
IC, interferential current; CI, confidence interval; RCT, randomized clinical trial; MD, mean difference, SMD, standard mean difference.
Quality of evidence:.
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect.
One study7 compared IC to no intervention. We found very low-quality evidence that the IC reduces pain and disability compared to no treatment. Estimated mean pain intensity after treatment (VAS): IC group:10 mm, and control group: 55 mm. Additional data could not be obtained as the authors did not respond to our emails.
Interferential current versus placeboPain intensity immediately post-treatmentSeven trials40–44,46,49 compared IC to placebo for pain intensity. We found moderate-quality evidence (downgraded for inconsistency) that IC probably reduces pain intensity compared to placebo (MD = −1.57 points in a 0–10 scale; 95% CI: −2.17, −0.98, n = 865), resulting in a moderate effect that is potentially clinically meaningful. A subgroup analysis showed that IC may reduce pain intensity with a single session (MD = −1.83 points in a 0–10 scale; 95% CI: −2.42, −1.24, n = 567) or after 12 to 18 sessions (MD = −0.60 points in a 0–10 scale; 95% CI: −1.13, −0.07, n = 298) compared to placebo (Fig. 2).
Disability immediately post-treatmentThree trials40–42 compared IC to placebo for disability. We found moderate-quality evidence (downgraded for imprecision) that IC probably reduces disability compared to placebo (MD = −1.51 points on a 0–10 scale; 95% CI: −2.57, −0.46, n = 403), resulting in a moderate effect that is potentially clinically meaningful (Supplementary material – Fig. S1).
Pain intensity and disability at intermediate-term follow-upTwo trials40,41 compared IC to placebo for pain intensity and disability at intermediate-term follow-up. We found moderate-quality evidence (downgraded for imprecision) that IC probably does not reduce pain intensity (MD = −0.02 points; 95% CI: −0.70, 0.65, n = 298) (Supplementary material – Fig. S2) and disability (MD = −0.72 points; 95% CI: −2.27, 0.83, n = 298) (Supplementary material – Fig. S3).
Interferential current versus other intervention (TENS and manual therapy)Pain intensity immediately post-treatment - Interferential Current versus tensFive trials7,38,47–49 compared IC to TENS. We found low-quality evidence (downgraded for risk of bias and inconsistency) that IC may reduce pain intensity compared to TENS (SMD = −0.32; 95% CI: −0.61, −0.03, n = 420) (Supplementary material – Fig. S4), resulting in an uncertain clinical meaningfulness effect given the lower boundary of the 95% CI near 0.
Pain intensity immediately post-treatment - interferential current versus manual therapyOne trial45 compared IC to manual therapy. We found very low-quality evidence that IC may reduce pain intensity compared to manual therapy (MD = −15.52 mm in a 0–40 mm scale; 95% CI: −24.89, −6.15, n = 64), resulting in a small effect of uncertain clinical significance given the width of the confidence interval.
Disability immediately post-treatment - interferential current versus tensThree trials6,7,38,48 compared IC to TENS. We found very low-quality evidence that IC may not reduce disability compared to TENS (MD = −0.67 points; 95% CI: −3.72, 2.38; n = 50) (Supplementary material – Fig. S5).36,46 One trial6 reported the data only in graphs. We found very low-quality evidence that IC may not reduce disability compared to TENS. Additional data could not be obtained as the authors did not respond to our emails.
Disability immediately post-treatment - Interferential Current versus manual therapyOne trial45 compared IC to manual therapy. We found very low-quality evidence that IC may reduce disability compared to manual therapy (MD = 13.38 points in a 0 – 100 scale; 95% CI: 4.97, 21.78, n = 64).
Interferential current plus other intervention versus other interventionPain intensity and disability immediately post-treatmentOne trial compared the additional effect of IC to massage.14 We found very low-quality evidence that IC plus massage may reduce pain intensity compared to massage alone (MD = −1.05 points; 95% CI: −1.87, −0.23, n = 62). We also found very low-quality evidence (downgraded for imprecision and inconsistency) that IC plus massage may improve disability compared to massage alone (MD = −3.01 points; 95% CI: −4.62, −1.40, n = 62). However, these results are small and probably not clinically meaningful.
One trial compared the additional effect of IC to exercises48 for pain and disability. We found very low-quality evidence that IC plus exercise does not reduce pain intensity (MD = −0.09 points; 95% CI −1.30, 0.48, n = 22) and disability (MD = −1.72 points; 95% CI −2.71, 1.65, n = 22) more than exercise alone.
DiscussionTo our knowledge, this is the first systematic review to evaluate the effectiveness of IC in patients with non-specific chronic low back pain, comparing IC versus: no intervention, placebo, other intervention (TENS and manual therapy), and the additional effect of IC when combined with exercises or massage. The most relevant findings of this systematic review showed evidence of moderate quality that IC probably reduces pain intensity and disability compared to placebo immediately post-treatment with moderate effect size. These potentially clinically meaningful results were found in studies that used the following parameters: a carrier frequency of 1, 2, or 4 kHz, an AMF of 2, 65, or 100 Hz, quadripolar technique, and from 1 to 18 sessions for 30 min per session. But, moderate quality evidence also showed that these findings were not sustained at intermediate-term follow-up. Then, similar to TENS,50 IC seems to be potentially effective for pain relief only immediately after the treatment.
To date, the physiological effects are not well-established.51 IC is thought to decrease the stimulation of cutaneous sensory nerves close to the electrodes while increasing the effect on deep tissues.11 In addition, some theories have been proposed to explain the analgesic effect, such as the gate control theory, descending pain suppression pathway, and physiological block.51 Another theory that should be considered is that medium-frequency currents, such as IC, are better for reducing skin impedance and increasing tissue penetration, consequently providing greater patient comfort9,10 possibly associated with greater effectiveness of pain relief.11 There is no experimental research investigating the mechanism of action of IC for pain relief, with claims based on the mechanism of action of TENS. Thus, studies are needed to investigate the analgesic mechanism of action of IC.
Our systematic review also showed low quality evidence that IC is better than other intervention in decreasing pain intensity immediately post-treatment in patients with chronic non-specific low back pain. However, this result should be interpreted with caution as the quality of the evidence was low, the effect size was small, and the heterogeneity was 56%. In contrast, a systematic review suggested that TENS and IC had similar positive effects on pain and disability.52 However, their systematic review was performed in patients with acute and chronic pain, and the methodological quality was considered very heterogenic.52
There were no significant results in the additional effect of IC for improving pain intensity (IC plus massage versus massage; IC plus exercises versus exercises)14 and disability (IC plus exercises versus exercises).47 The evidence was of low quality and the meta-analyses were performed with only two trials. We believe that more pragmatic studies, with larger samples and high methodological quality, that compare or add IC to a guideline-based treatment are necessary.
This review has a number of strengths. There was no restriction of language for the included studies, thus minimizing the risk of language bias.53 This review followed all recommendations of the Cochrane Handbook for systematic reviews of interventions.54 The quality of the evidence was carefully assessed according to GRADE recommendations,55 in which the quality of evidence is assessed and consequently the quality of confidence in the results. A highly sensitive search strategy was used to identify studies in the main databases and clinical trial registries, complemented by a manual search in studies relevant to the topic. However, it is possible that some studies were published in local databases and consequently were not found and included in this review, which may be considered a potential limitation of the review.
ConclusionEvidence of moderate-quality shows that interferential current seems to be more effective than placebo for improving pain intensity and disability in patients with chronic non-specific low back pain. Future trials are needed to investigate the IC efficacy comparing to other interventions and when combined to other interventions.
Mauricio A. Luz Júnior was supported by a PhD scholarship from São Paulo Research Foundation17/08659-2.