To investigate clinical outcomes according to ventilatory support indication in subjects with chronic obstructive pulmonary disease exacerbation in a “real-life” Emergency Department and to analyze potential predictors of successful noninvasive positive pressure ventilation.
MethodsRetrospective cohort performed over an 18-month period, comparing the following patient groups with chronic obstructive pulmonary disease exacerbation: Group A composed of patients initially selected to receive noninvasive positive pressure ventilation without the subsequent need for invasive mechanical ventilation (successful-noninvasive positive pressure ventilation); Group B composed of patients transitioning from noninvasive positive pressure ventilation to invasive mechanical ventilation (failed-noninvasive positive pressure ventilation); and Group C composed of patients who presented with immediate need for invasive mechanical ventilation (without prior noninvasive positive pressure ventilation).
Results117 consecutive chronic obstructive pulmonary disease exacerbation admissions (Group A=96; Group B=13; Group C=8) of candidates for ventilatory support were reviewed. No differences in baseline disease severity and physiological parameters were found between the groups at Emergency Department admission. Nevertheless, Group B had higher intensive care unit admission, length of hospital stay, length of intensive care unit stay, and higher in-hospital mortality compared to Group A. Group C also had worse outcomes when compared to Group A. The only independent variable associated with the successful use of noninvasive positive pressure ventilation were improvement in arterial carbon dioxide pressure after 1h of noninvasive positive pressure ventilation use and its tolerance.
ConclusionOur data confirmed in a “real life” Emergency Department cohort that successful management of chronic obstructive pulmonary disease exacerbation with noninvasive positive pressure ventilation showed lower in-hospital mortality and Intensive Care Unit stay when compared to patients transitioning from noninvasive positive pressure ventilation to invasive mechanical ventilation or patients who presented an immediate need for invasive mechanical ventilation. noninvasive positive pressure ventilation tolerance and higher arterial carbon dioxide pressure reduction after 1-h of noninvasive positive pressure ventilation were predictors of successful treatment. These results should be confirmed in a prospective randomized controlled trial.
Exacerbations of chronic obstructive pulmonary disease (ECOPD) are clinically important events in the course of the disease,1 representing a great burden of morbidity and mortality.2,3 Standard medical care consists of supplemental oxygen, bronchodilators, systemic corticosteroids, and antibiotics.1 Noninvasive positive pressure ventilation (NIPPV) is the recommended treatment for hypercapnic ECOPD after optimal medical management.4 NIPPV is supported by a number of randomized controlled trials (RCT) that have shown a reduction in intubation rates,5–7 mortality,5,6,8 and length of hospital stay.5,7 As a consequence, the use of NIPPV for ECOPD has increased steadily in the last decade whereas invasive mechanical ventilation (IMV) use has declined.9,10 This was accompanied by an increase in the absolute number of patients transitioning from NIPPV to IMV, whose in-hospital mortality appeared to be worsening over time.10 In previous research, concerns were raised that NIPPV might delay intubation leading to a worse outcome.11,12 On the other hand, a dedicated NIPPV team was associated with a lower risk of death or intubation in subjects with ECOPD.13 Therefore, we decided to examine the outcomes of subjects with ECOPD treated according to current recommendations in a “real-life” scenario. In this context, subjects might have different responses to treatment not captured in traditional RCT which usually evaluate the performance of an intervention under ideal and controlled circumstances.14
The Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (GOLD)1 has presented recommendations for hospital and intensive care unit (ICU) admission, NIPPV and IMV. However, less evidence is available on their use in routine practice in the emergency department (ED) setting in Brazil, a practice that is becoming more common with increasing participation of physical therapists.
Therefore, the aim of the present study was to compare clinical outcomes according to initial ventilatory support indication in subjects with ECOPD in a “real-life” ED and investigate predictors of successful NIPPV. Secondly, baseline COPD severity and physiological parameters at ED admission were compared in patients initially selected to receive NIPPV without the subsequent need for IMV (Group A: successful NIPPV), those transitioning from NIPPV to IMV (Group B: failed NIPPV), and patients who presented an immediate need for IMV (Group C).
MethodsStudy designThe study involved evaluation of a retrospective cohort consisting of consecutive subjects treated in a single ED at a Brazilian tertiary care teaching hospital over an 18-month period (May 2012 to November 2013). The study was approved by the Institutional Ethics Committee (No. 14-0210/13; Hospital de Clínicas de Porto Alegre Research Ethics Committee, Porto Alegre, RS, Brazil) and informed consent was waived due to its retrospective nature.
ParticipantsPotential subjects were identified according to the International Classification of Diseases (ICD-10) (codes J41.0; J41.1; J41.8; J43.0, J43.1, J43.2, J43.8; J44.0, J44.1, J44.8, J44.9; and J96) and attending at our ED during the study period. Main inclusion criteria were a confirmed diagnosis of COPD based on clinical data and previous spirometry (post-bronchodilator forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC)<0.7)1 and the presence of severe ECOPD with acute respiratory failure requiring ventilatory support. In those subjects presenting more than one ECOPD, only the first event was considered in the study. All data were obtained from an electronic medical record system (AGHweb®; HCPA, Porto Alegre; Brazil) which contained the full medical history of those attending the institution; therefore, a complete follow-up of all subjects was available.
Data collectionThe following data were collected: demographics (i.e. age, gender); prior medical records at the institution (i.e. spirometry values, co-morbidities, smoking history, previous use of home oxygen therapy and home NIPPV); features of current exacerbation (i.e. vital signs, Manchester protocol for risk classification values,15 arterial blood gases (ABG) analyses); outcomes (i.e. need for endotracheal intubation/IMV, length of hospital stay, need for ICU admission, length of ICU stay, in-hospital mortality; and 1-year mortality).
Vital signs, respiratory frequency (f), heart rate (HR), mean blood pressure (MBP), oxyhemoglobin saturation by pulse oximetry (SpO2); fraction of inspired oxygen (FiO2), and ABG measurements were recorded at admission and 1h after ventilatory support implementation. NIPPV intolerance was defined as its interruption due to subject refusal to continue because of discomfort or an intolerable sensation of breathlessness, even after attempting intermittent use.16
ECOPD management protocolECOPD was defined as an acute event characterized by a worsening of the subject's respiratory symptoms, reaching excessive levels when compared to normal day-to-day variations and leading to changes in medication.1
The ED team followed a standardized protocol based on recommendations from the GOLD executive summary1 for hospital admission, ICU and IMV indication (respectively, Table 6, 8 and 10 of reference 1). The indications for NIPPV in the context of ECOPD are one or more of the following: (1) respiratory acidosis (arterial pH≤7.35 and/or PaCO2≥45mmHg) and/or; (2) severe dyspnea with clinical signs of respiratory muscle fatigue and/or increased work of breathing.1
The protocol was developed and applied by a multidisciplinary team including emergency physicians, physical therapists, and nurses. The physical therapy-driven NIPPV management included a prospective daily collection of clinical and laboratory data. Once indicated, early initiation of NIPPV after ED admission using a BiPAP®Vision® Ventilatory Support System (Philips; Respironics; Andover, USA) in S/T-mode was performed. A facial mask (Respironics PerforMax, Philips) was used, adjusted to the size of the subject and held in place with straps. Ventilatory settings were adjusted considering the subject's clinical status, breathing pattern, SpO2 and ABG measurements. The inspiratory positive airway pressure (IPAP) was adjusted to achieve a tidal volume of at least 8–10mL/kg of predicted weight as tolerated.17 Expiratory positive airway pressure (EPAP) was set at 5cm H2O7 and increased in increments of 2–3cmH2O up to 10–12cmH2O in order to assure a SpO2 of at least 90% with the lowest FiO2 possible. All subjects were continuously monitored with electrocardiography and pulse oximetry.
Statistical analysisThe sample size was calculated based on the proportion of non-survivor subjects with ECOPD receiving successful NIPPV (9%) vs. patients transitioning from NIPPV to IMV (27%),10 considering a sampling ratio of 4:1, a type II error of 20% and a type I error of 5%. This resulted in 53 patients for group A and 13 patients for group B. We included all consecutive patients admitted at our ED who met the inclusion criteria since May 2012 until achieving the minimal number of subjects in each group.
Continuous data were expressed as mean and standard deviation or median (range) according to distribution while categorical data as a number (%). Comparisons among the three groups were performed using Analysis of Variance (ANOVA) with the post hoc Bonferroni or Kruskal–Wallis test according to distribution. Chi-Square test was used for categorical parameters and Z test with Bonferroni correction for post hoc comparisons. Physiological comparisons at admission between Groups A and B were performed with the Student t test or Mann–Whitney U test according to distribution. Similarly, variables pre and 1-h post-intervention were compared with the paired Student t test or Wilcoxon signed-rank test.
Logistic Regression Analysis was used to predict successful NIPPV. Variables showing a p<0.1 in univariate analyses entered in the multivariate model. Thereafter, a receiver operating characteristic curve (ROC) analysis was used to evaluate the performance of significant continuous independent parameters. Poisson Regression Analysis with robust variance was conducted to examine whether the subsequent need for IMV was associated with in-hospital mortality risk in subjects on initial NIPPV support. The SPSS statistical package (v.22.0.0.1, Chicago, USA) was used for the statistical analyses. The level of statistical significance was set at p<0.05.
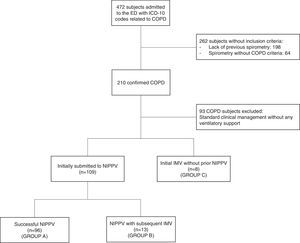
Results472 subjects were admitted to the ED with ICD-10 codes related to COPD over the 18-month study period. Of these, there were 93 subjects without the need for ventilatory support, 198 without a previous spirometry test, and 64 patients who had a previous spirometry test without a COPD diagnosis who did not meet the inclusion criteria. NIPPV was used in 109 of the 117 subjects (93%) who required ventilatory support at admission, while the remaining subjects (8/117; 7%) received initial IMV without prior NIPPV (Group C). Among the 109 subjects receiving NIPPV, 13 (12%) required transition from NIPPV to IMV (Group B) (Fig. 1). The difference in previous functional and clinical characteristics, domiciliary oxygen therapy, and continuous NIPPV, as well as Manchester risk classification at ED admission were not statistically significant between Groups A (successful NIPPV) and B, yet in Group C, an increased FEV1/FVC ratio was found (Table 1). Notwithstanding these findings, Group B showed worse clinical outcomes: higher ICU admission, longer length of hospital stay, longer length of ICU stay, and greater in-hospital mortality (Table 2). Poisson regression analyses showed that Group B had a 245% higher risk of mortality when compared to Group A (Exp(B): 3.45; 95% CI: 1.74–6.84; p<0.001). In addition, subjects initially subjected to IMV (n=8) showed higher in-hospital mortality (4/8; 50%) and length of ICU stay (15.8 SD 13.3 days) when compared to Group A (p<0.05), however, no significant difference from Group B was reported (Table 2).
Baseline demographic data and clinical characteristics of 117 patients with an initial indication for NIPPV support.
| Group A (n=96) | Group B (n=13) | Group C (n=8) | |
|---|---|---|---|
| Male | 49 (51) | 7 (54) | 5 (50) |
| Age, yrs | 68 (37–85) | 61 (56–78) | 67 (54–80) |
| Lung function | |||
| FEV1, L | 0.81 (0.39–2.34) | 0.65 (0.54–2.20) | 1.04 (0.77–1.58)*,† |
| FEV1, %pred | 35±13 | 34±15 | 43±4 |
| FVC, L | 1.85 (0.93–3.59) | 1.45 (1.41–3.54) | 1.78 (1.03–2.52) |
| FVC, %pred | 59 (26–86) | 53 (39–79) | 61 (21–68) |
| FEV1/FVC | 0.46±0.10 | 0.45±0.86 | 0.64±0.05*,† |
| GOLD classification | |||
| Moderate (II) | 19 (20) | 1 (8) | 1 (13) |
| Severe (III) | 51 (53) | 5 (38) | 5 (62) |
| Very severe (IV) | 26 (27) | 7 (54) | 2 (25) |
| Comorbid conditions | |||
| Hypertension | 57 (59) | 8 (62) | 6 (75) |
| Ex-smoker | 56 (58) | 7 (54) | 5 (62) |
| Active smoking | 16 (17) | 4 (31) | 2 (25) |
| Diabetes mellitus | 23 (24) | 6 (46) | 3 (38) |
| Heart failure | 19 (20) | 2 (15) | 3 (38) |
| Manchester risk classificationa | |||
| Immediate | 17 (18) | – | 2 (25) |
| Very urgent | 66 (69) | 13 (100) | 5 (62) |
| Urgent | 5 (5) | – | 1 (13) |
| Home NIPPV use | 5 (5) | 0 (0) | 0 (0) |
| Home oxygen therapy | 42 (44) | 3 (23) | 1 (13) |
Values are presented as mean±SD or median (minimum–maximum), and absolute number (frequency %). Definition of abbreviations: NIPPV, noninvasive positive pressure ventilation; FEV1, forced expiratory volume in 1s; %pred, percent of predicted; FVC, forced vital capacity.
Clinical outcomes comparing 117 subjects with successful NIPPV (Group A), initial NIPPV with subsequent transition to invasive mechanical ventilation (Group B) and initial indication to IMV (Group C).
| Group A (n=96) | Group B (n=13) | Group C (n=8) | |
|---|---|---|---|
| Need for ICU | 46 (48) | 13 (100)* | 7 (88) |
| Length of ICU stay, days | 0 (0–102) | 12 (2–49)* | 15 (0–44)† |
| Length of hospital stay, days | 15(3–105) | 28 (10–52)* | 24 (1–74) |
| In-hospital mortality | 18 (19) | 7 (54)* | 5 (63)† |
| 1-year mortality | 9 (12) | 0 (0) | 0 (0) |
Values are presented as mean±SD or median (minimum–maximum), and absolute number (frequency %). Definition of abbreviations: NIPPV, noninvasive positive pressure ventilation; ICU, intensive care unit.
At admission, HR was the unique parameter higher in Group B compared to Group A (Table 3). After 1h of NIPPV, f, HR and SpO2 significantly improved in both groups while ABG parameters (i.e. PaCO2, bicarbonate, and pH) improved only in Group A (Table 3). Furthermore, NIPPV tolerance was significantly higher in this group: 85/96 (88%) vs. 8/13 (61%) (p=0.02).
Physiological parameters before and after 1h of NIPPV for 117 subjects.
| Group A (n=96) | Group B (n=13) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| f, breaths/min | 30 (16–54) | 20 (14–38)† | 34 (22–44) | 20 (13–32)* |
| HR, beats/min | 110±20§ | 97±15† | 125±16 | 103±15* |
| SpO2, % | 90 (54–100) | 97 (87–100)† | 87 (69–100) | 98 (92–100)* |
| FiO2, % | 36 (21–100) | 35 (21–80)† | 32 (21–100) | 35 (25–80) |
| MBP, mmHg | 97 (49–170) | 90 (51–143)* | 89 (57–159) | 99 (71–108) |
| ABG | ||||
| PaCO2, mmHg | 70.3±23.6 | 55.1±15.7† | 62.7±21.0 | 52.72±15.7 |
| HCO3, mm/L | 33.3±9.1 | 30.9±6.9† | 32.9±7.7 | 30.6±6.5 |
| pH | 7.30±0.11 | 7.37±0.08* | 7.34±0.10 | 7.39±0.08 |
| PaO2/FiO2 ratio | 184 (48–500) | 228 (114–355) | 179 (48–350) | 250 (74–420) |
Values are presented as mean±SD or median (minimum–maximum). Definition of abbreviations: RR, respiratory rate; HR, heart rate; SpO2, oxygen saturation by pulse oximetry; FiO2, fraction of inspired oxygen; MBP, mean blood pressure; ABG, arterial-blood gases; PaCO2, arterial partial pressure of carbon dioxide; HCO3, bicarbonate; PaO2, arterial pressure of oxygen; PaO2/FiO2 ratio, arterial partial pressure of oxygen/fraction of inspired oxygen ratio.
No clinical or physiological variables at admission were significant predictors in subjects transitioning from NIPPV to IMV. In fact, improvement in PaCO2 after 1h of noninvasive ventilation (ΔPaCO2) and tolerance to NIPPV were the only independent variables related to NIPPV success (Table 4). A ROC curve analysis identified an optimal threshold of 6.5mmHg reduction in PaCO2 (after 1h of NIPPV) to avoid IMV with a sensitivity of 87% and specificity of 71% (area under the curve=0.77, SD=0.09; p=0.02).
Variables related to noninvasive positive pressure ventilation success (i.e. avoidance of invasive mechanical ventilation).
| Values | Univariate test p value | Multivariate logistic regression | |||
|---|---|---|---|---|---|
| β | Odds ratio (CI 95%) | p value | |||
| ΔPaCO2, cmH2O | −13.12±16.78 | 0.032* | 0.873 | 0.13 (0.77–0.99) | 0.03† |
| ΔPaO2/FiO2 ratio | 36.5±126.3 | 0.713 | – | – | – |
| Δf, breaths/min | −11±7 | 0.676 | – | – | – |
| ΔHR, beats/min | −15±17 | 0.113 | – | – | – |
| NIPPV intolerance | No | 0.001* | 0.007 | 0.99 (0.00–0.40) | 0.01† |
| Home oxygen therapy | Yes | 0.357 | – | – | – |
| FEV1% | 33±13 | 0.588 | – | – | – |
Values presented as mean±SD or categorically defined. Definition of abbreviations: Δ, values after 1h of NIPPV minus values at the beginning; PaCO2, arterial partial pressure of carbon dioxide; PaO2/FiO2 ratio, arterial partial pressure of oxygen/fraction of inspired oxygen rate variation.
Our study evaluated clinical outcomes on subjects with ECOPD in a real practice ED setting according to ventilatory support indication based on current recommendations.1 The main findings of the present study were: (1) subjects who failed after an initial NIPPV attempt (Group B) presented higher ICU admission, in-hospital mortality, length of hospital stay and length of ICU stay compared to subjects with successful NIPPV (Group A); (2) although subjects in Group B were significantly more tachycardic when compared to Group A, there were no identifiable physiological parameters at admission to predict a transition from NIPPV to IMV. In fact, the significant parameters for predicting NIPPV failure were intolerance to NIPPV and lower reduction in PaCO2 (i.e. parameters available only after administration of ventilatory support); (3) no difference in prior clinical characteristics were found between Groups A and B, including COPD severity, previous home oxygen therapy and prevalence of comorbidities. Nonetheless, it is important to highlight that because of the size of our study, further research may be necessary to detect significant differences in baseline characteristics between Groups A and B. The use of NIPPV has revolutionized the management of ECOPD18 with a shift from invasive to noninvasive ventilation. Therefore, it is considered reasonable the description of the effectiveness of any proven intervention in a real-life scenario where disease severity, comorbidities and the real practice of health assistance may interfere with outcomes. Furthermore, the study of a specific scenario (in this case, a Brazilian tertiary care ED) is useful for the local evaluation of quality assistance, comparison with other institutions as well as present local/regional results. Our data showed a higher percentage of subjects who required a transition from NIPPV to IMV (13/109; 12%) when compared to a report from an extensive database including over 7.5 million ECOPD admissions (5%)10 or a recent observational study (8%).19 Nonetheless, another extensive database study including more than 723,000 ECOPD hospitalizations reported a 17% NIPPV failure rate9 while another recent observational study showed a 20% rate of those patients transitioning from NIPPV to IMV.20 Therefore, our figures show a NIPPV failure rate within the literature range.
In addition, as previously reported,10 the study found that subjects transitioning from NIPPV to IMV (Group B) presented significantly worse clinical outcomes. Nevertheless, they were not significantly different from those patients initially receiving IMV since initial ED admission (Group C). Further studies are necessary to support this finding because a larger sample is necessary to detect differences between Groups B (n=13) vs. C (n=8) and a type II error could be present. In fact, the above-mentioned extensive database with millions of ECOPD admissions10 suggested that patients who failed NIPPV and transitioned to IMV experienced significantly higher mortality than patients who were immediately submitted to IMV, in addition to the longest and most expensive hospitalizations for COPD exacerbations. Finally, as expected and previously described,21 NIPPV was associated with better results than IMV for ECOPD in a real-world setting.
The identification of predictors of NIPPV failure is not a new concept, and it has been the focus of several previous works resulting in variable findings.16,18,22–24
Unfortunately, no variable capable of predicting NIPPV failure at admission was found. In fact, the only parameters obtained after 1h of NIPPV treatment such as improvement in PaCO2 levels and interface tolerance were significantly associated with success. Subjects who were recommended for noninvasive ventilation (and without a contra-indication) should be re-evaluated after 1h of ventilatory support. A PaCO2 reduction higher than 6.5mmHg was identified as the optimal threshold for success. Although some seminal pioneer RCTs demonstrating the clinical benefits of noninvasive ventilation in ECOPD did not show a significant difference in PaCO2 reduction compared to standard therapy,6,7 other studies have demonstrated a significant higher PaCO2 reduction associated with NIPPV.5,8 Accordingly, the overall combination of seminal RCTs18 and current observational studies19,24 demonstrated higher PaCO2 values after 1h of noninvasive ventilation in the usual medical care group and in the group who subsequently needed IMV, respectively. In fact, the optimal threshold identified in the present study to predict NIPPV success resembled the ∼9mmHg difference in PaCO2 reduction between groups (i.e. NIPPV vs. control) in a previous classical RCT.8 Therefore, NIPPV intolerance and/or absence of reduction in PaCO2 should be considered as meaningful predictors when considering the early transition from NIPPV to IMV in ECOPD. This information has important clinical implications: while a subject with higher likelihood of NIPPV failure with subsequent IMV is best managed in the ICU, a subject who is likely to be successfully treated with noninvasive ventilation can be appropriately managed in less intensive settings. Furthermore, tachycardia at baseline16,19 and persistence of tachycardia after 1h of noninvasive ventilation treatment19 were previously identified as independent predictors of NIPPV failure. Accordingly, despite not remaining in our multivariate analysis (possibly due to our underpowered sample), HR at baseline was significantly higher in patients with NIPPV failure (Table 3). The presence of tachycardia at admission might represent a possible marker of NIPPV failure and deserves further investigation. Finally, low pH value at admission, usually identified as NIPPV failure predictor19,24 was not a significant independent predictor in our multivariate analysis, in accordance with another study showing that ECOPD with severe acidosis (pH≤7.25) could be successfully treated with NIPPV at intermediate care units.25
The strength of the present approach is that all COPD cases were confirmed using spirometry (Fig. 1), whereas in the majority of previous studies5,6,19,21,26–28 this approach was not used for the COPD diagnosis.1 Furthermore, other clinical conditions than COPD that could be responsible for acute respiratory failure and represented potential confounding elements in previous works were not included.29–32 One of the few studies describing spirometric measurements in nearly 90% of the subjects found significantly lower FVC in patients with NIPPV failure,22 which was not observed in our study. Nevertheless, a low statistical power due to the sampling cannot be ruled out. In addition, this study has intrinsic limitations due to its retrospective nature besides the higher probability of protocol violations in real practice and emergency settings. These aspects justify the lack of some potential interesting analyses such as hospital admission comparison among groups (i.e. all patients were admitted to the hospital) and the effect of NIPPV length prior IMV in Group B (due to lack of trustworthy registers).
In conclusion, COPD patients with subsequent transition to IMV (Group B: NIPPV failure) had higher ICU admissions, longer ICU stays, and greater in-hospital mortality compared to those who had successful NIPPV (Group A) in a real practice ED scenario. NIPPV tolerance and higher PaCO2 reduction after 1h of NIPPV were the only predictors of successful NIPPV. These results should be further confirmed in a randomized controlled trial.
Conflicts of interestThe authors declare no conflicts of interest.