Public safety workers (PSW), such as police officers and firefighters, face a high prevalence of chronic low back pain (LBP). Effective and accessible interventions are essential to improve their health and job performance.
ObjectiveTo evaluate the effectiveness of a m-health-based core stability exercise self-management program combined with health education compared to m-health-based health education alone in PSW with chronic LBP.
MethodsForty-seven PSW with chronic LBP were randomly assigned to receive a smartphone app-based self-managed exercise program (twice a week) plus health education (INT; n = 23) or health education alone (CON; n = 24) for eight weeks. Primary outcomes were pain intensity and disability at 8 weeks. PSW were assessed preintervention and 8 and 16 weeks after the randomization.
ResultsThose in the INT group had a greater reduction in pain intensity (MD = -1.54; 95 %CI -2.95, -0.13) and disability (MD = -3.23; 95 %CI -5.51, -0.95) than those in the CON group at 8 weeks. Quality of life, self-efficacy, and anxiety improved for those in the INT group compared to those in the CON group at eight weeks. The treatment effects remained for disability, quality of life, and anxiety in the follow-up period of 16 weeks. No between-group differences were found for depression, stress, sleep quality, or neuromuscular outcomes.
ConclusionA m-health-based core stability exercise self-management program combined with health education was more effective in reducing pain intensity, disability, and anxiety, as well as improving quality of life and self-efficacy, compared to m-health-based health education alone in PSW with chronic LBP.
Low back pain (LBP) is a pervasive global health issue, the leading cause of disability-adjusted life years, and a significant hindrance to workforce productivity.1,2 Chronic LBP persists beyond 12 weeks3 and affects about one-fifth of the global adult population,4 with particularly high prevalence in occupational sectors, notably among public safety workers (PSW), such as police officers and firefighters.5 These professionals experience chronic LBP rates ranging from 28.7 % to 76.2 % worldwide.6–8
Extensive evidence highlights the role of poor general health, physical demands, and psychological stress as key risk factors for LBP.9 PSW are frequently engaged in activities involving sustained awkward postures, heavy lifting, unconscious victim rescue, and are exposed to distressing situations, making them more prone to chronic LBP.10,11 While these risk factors are not often modifiable, exercise interventions focusing on accessibility and core strengthening through technology, have shown promise in mitigating LBP.12–15
Digital interventions, particularly mobile health (m-health) applications, offer a promising avenue to overcome traditional barriers faced by PSW for face-to-face interventions (e.g., time, distance, and availability).16,17 Given the significant occupational risks and high prevalence of LBP in this population, effective and accessible interventions are critical.
This study aimed to investigate the efficacy of a m-health-based core stability exercise self-management program combined with health education on reducing pain and disability compared to m-health-based health education alone for managing chronicLBP in PSW.
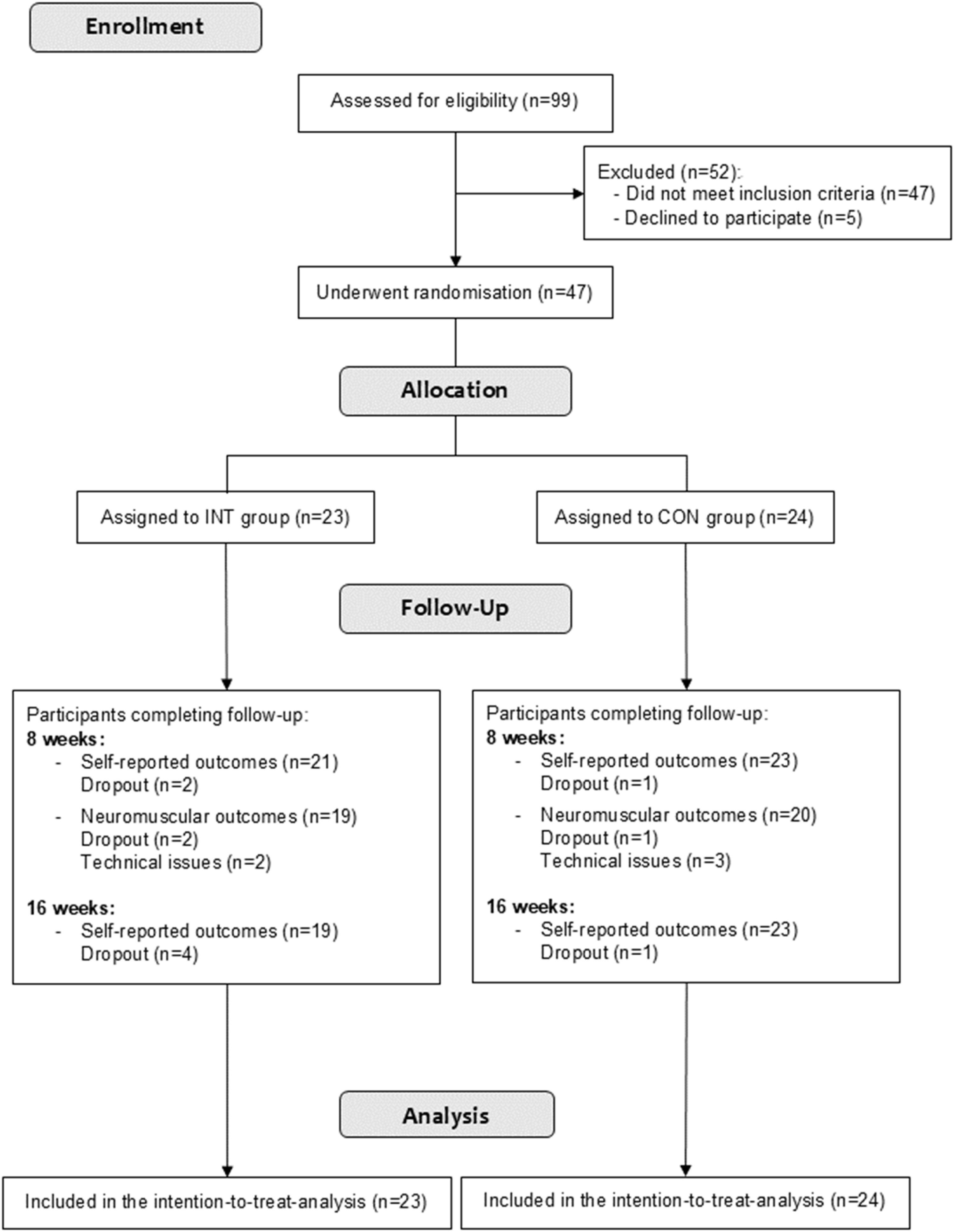
MethodsStudy designThis randomized controlled trial was conducted at the Universidade Federal de Pelotas (UFPel) and the Sindicato dos Policiais Rodoviários Federais do Rio Grande do Sul, Brazil (Fig. 1). The study was approved by the Research Ethics Committee of the Escola Superior de Educação Física at UFPel (protocol 58,715,422.3.0000.5313), and followed the CONSORT guidelines.18,19 All individuals provided signed informed consent before participation. The trial was prospectively registered on ClinicalTrials.gov (NCT05481996), and the full protocol is available online.20
ParticipantsWe enrolled actively working PSW (police officers and firefighters working in public security organizations in Rio Grande do Sul state), aged 18–60, with chronic LBP (Numerical Rating Scale score ≥3/10).21 All participants were engaged in either operational or administrative duties at the time of enrollment. Participants had to have an Android smartphone and email access. Chronic LBP was defined as discomfort between the 12th ribs and lower gluteal folds, lasting for >12 weeks, without a specific diagnosis.22 Exclusions included neurological symptoms, severe spinal diseases, cardiovascular/metabolic conditions, recent spine surgery (over the last 12 months), pregnancy, recently receiving physical therapy or performing exercise (strength training for core muscles, Pilates, yoga) within the last three months, and exercise contraindications. The Portuguese version of the Physical Activity Readiness Questionnaire (PAR-Q) was used to pre-screen for physical activity participation at baseline to ensure suitability and safety for engaging in the exercise program.23 Participants were recruited via social media advertising, local newspapers, and professional email from public security organizations from October 2022 to May 2023.
ProceduresThe lead researcher conducted video call interviews to assess eligibility criteria. Eligible participants received consent and standardized forms via email. A second video call with a blinded assessor collected baseline data before randomization. In-person neuromuscular assessments followed. Participants received a yoga mat and elastic band post-assessment. Assessors were uniformly trained to administer questionnaires and neuromuscular tests.
OutcomesSelf-reported outcomes were evaluated via video call by a blinded assessor at baseline, post-treatment (eight weeks), and at 16-weeks (follow-up). Neuromuscular outcomes at baseline and post-treatment were assessed in-person by four blinded assessors, who remained uninformed about participant allocation or treatment details.
Primary outcome measuresPrimary outcomes were pain intensity and disability measured post intervention (i.e., at 8 weeks post-randomization). The NRS quantified average pain intensity over the last 7 days, ranging from 0 (no pain) to 10 (worst pain).21 The Roland Morris Disability Questionnaire assessed LBP-related disability using a 24-item functional assessment, with higher scores indicating more significant disability.24,25
Secondary outcome measuresPain intensity and disability were assessed as secondary outcomes at 16 weeks post-randomization. Pain-related quality of life was evaluated using the WHOQoL-Pain,26 while self-efficacy was measured with the Chronic Pain Self-Efficacy Scale.27 Higher scores on both instruments indicate better quality of life and self-efficacy. Depression, anxiety, and stress symptoms were assessed using the Depression Anxiety Stress Scale,28 and sleep quality was measured with the Pittsburgh Sleep Quality Index.29 Higher scores on these scales indicate more severe symptoms. Work ability was evaluated with a single-item question regarding any change in job demands due to back and/or leg pain.30,31
Isometric trunk flexor and extensor endurance were measured through a test of sustained isometric contraction, where longer durations indicate better endurance.32–34 The maximum isometric strength of trunk extensors and flexors (Maximum Voluntary Isometric Contraction—MVIC) was assessed using a calibrated load cell (Miotec®, Porto Alegre, Brazil) of 200 kgf. Muscular activation of spine flexors (rectus abdominis) and extensors (erector spinae-longissimus) was measured simultaneously to the MVIC using surface electromyography (sEMG). The protocol description and neuromuscular assessment illustration are available in the Supplementary material.
Randomization and blindingDuring the initial phase (September to December 2022), eligible participants were randomly assigned to either the intervention (INT) or control (CON) group in a 1:1 ratio. However, this initial simple randomization resulted in uneven group sizes, leading to a second randomization phase (March to August 2023) to balance participant numbers across treatment arms. The second randomization involved two initial blocks with a size of 8 and a ratio of 2:1 (control: intervention) to address group imbalances, followed by 6 blocks of size 4 and a final block of size 6, all with a 1:1 ratio. Both randomization phases utilized computer-generated methods. Allocation sequences were arranged by an independent investigator and concealed in sequentially numbered, sealed, opaque envelopes. Following randomization, participants received login credentials via email to access the designated application (app) version.
Given the nature of the intervention, participants and treatment providers could not be blinded. However, outcome assessors remained blinded to treatment allocation. Participants were instructed not to disclose their assigned groups or discuss interventions during outcome assessments, and no instances of unblinding occurred.
InterventionsWe adhered to the TIDieR (Template for Intervention Description and Replication) checklist guidelines for telehealth interventions in clinical trials.35 The interventions used a smartphone web app for eight weeks ("MY SAFE BACK"), developed by two Computer Science Ph.D. candidates under the supervision of a professor with a decade of software development experience. The app was developed using the Adonis framework, integrated with Firebase, and utilized MySQL for the database. Participants were instructed to self-manage the functions available in the app interfaces.
Participants were allocated to one of two app versions: m-health-based core training exercise and health education (INT); or m-health-based health education (CON). Before the intervention began, participants received a message and a video tutorial on how to install and self-manage the functions available in the app (https://mysafebackapp.web.app/dashboard).
INT group: m-health-based core training exercise and health educationThe "MY SAFE BACK" app interface for the INT group featured four sections on the main screen: 1) an online booklet, 2) weekly messages, 3) contact support, and 4) an eight-week physical exercise program (Supplementary material - Fig. 2).
The online booklet contained general information on self-managing chronic LBP. Weekly messages included content from the booklet, offering insights on exercise benefits, motivation, and positive coping strategies for pain management (Supplementary material - Fig. 3). Additionally, participants had direct access to contact the researcher for app-related queries or to report any technical issues.
The exercise component (app exercises function) comprised 16 training sessions spread across eight weeks, with participants undertaking two sessions per week on non-consecutive days (training program provided in Supplementary material - Fig. 4). Developed by professionals with over five years of clinical expertise in chronic pain management through exercise, the program targeted static core muscle endurance and dynamic core stability improvement. We structured the regimen with a progressive training approach, adhering to established protocols for chronic LBP treatment (Supplementary material - Table 1 for training progression details).36–39
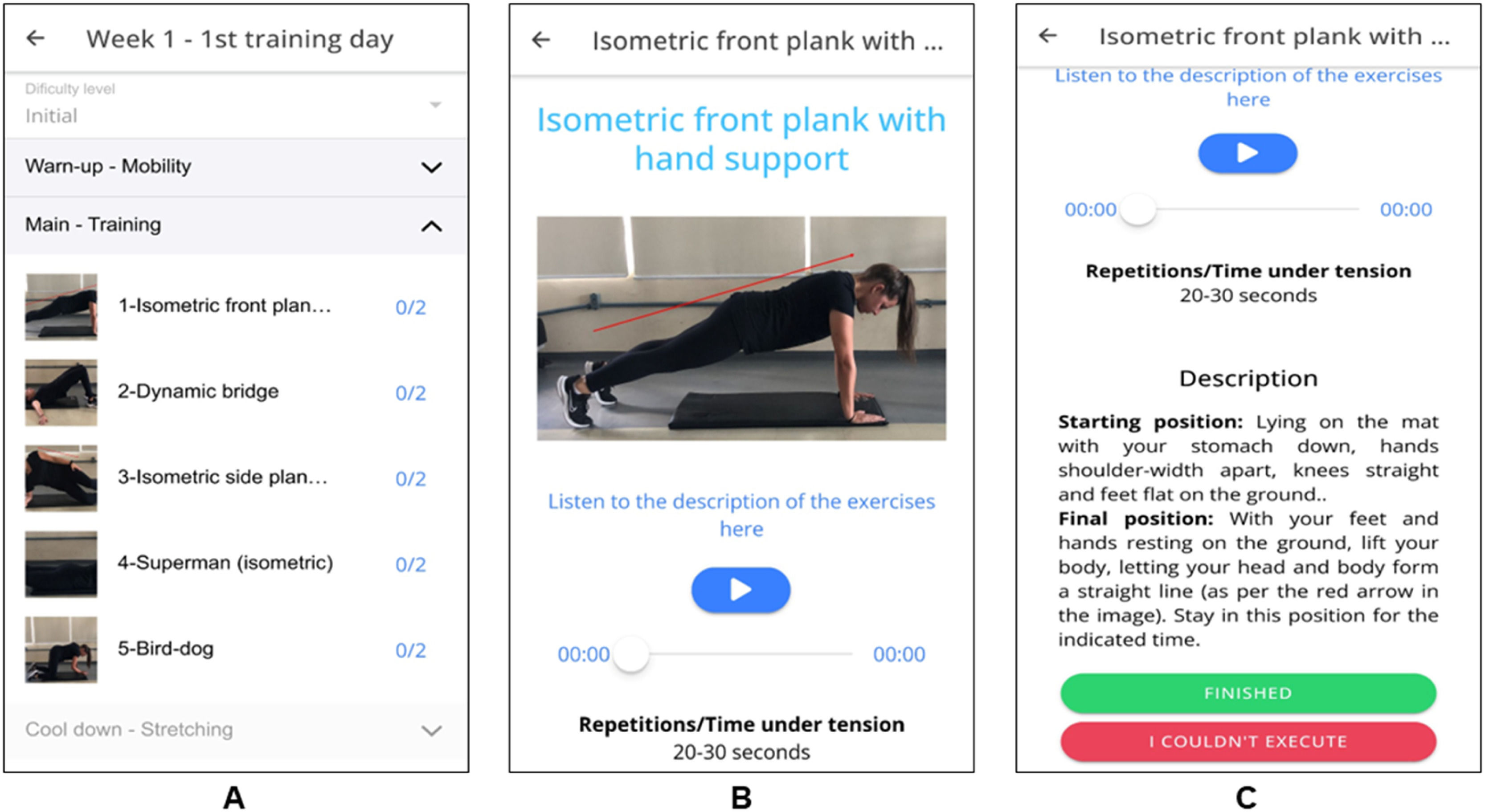
The training sessions were divided into three segments: warm-up (5 min), main session (20–25 min), and cooldown (5 min) (Fig. 2– Panel A). The main portion was conducted in a circuit format, with a consistent 30-second rest interval between exercises and an identical sequence in every session. A comprehensive list featuring illustrations of exercises performed at each stage is available in Supplementary material - Table 2. Following each exercise session, participants were prompted to assess their overall perceived exertion using the Borg scale (ranging from 0 to 10 points) displayed on the app.40
The app offered illustrated instructions using animated Graphics Interchange Format and descriptions and audio guidance for executing each exercise (Fig. 2– Panels B and C). Repetition counts or time under tension for each exercise were provided with target ranges (e.g., 10 to 12 repetitions, 20 to 30 s). Participants were prompted to indicate within the app if they could not meet these targets. All exercise sessions were conducted remotely and self-directed by the participants, with each week's sessions accessible through the app.
The app provided a count and progression bar ( %) of the number of completed training days to improve study adherence (Supplementary material - Fig. 4 - Panel A). Participants also received a text message in week 4 to encourage them to keep doing the exercises and ask questions whenever they had.
Adverse events were systematically monitored through the app starting from the second week of the intervention. At the beginning of each week, participants were prompted within the app to answer two questions: "Did you experience any new symptoms or worsening of existing symptoms this past week?" If they responded "yes," a follow-up question was displayed: "Do you think the treatment caused this? (yes/no)." Reports of adverse events were immediately reviewed by two independent researchers, and, when necessary, medical experts were consulted to determine the potential relationship to the intervention and appropriate measures to address the issue. Participants could have a video call with a physical therapist to assess issues or consider stopping treatment if it was believed to cause harm.
CON group: m-health education"MY SAFE BACK" main screen for those in the CON group included three subunits: 1) an online booklet with self-management information on chronic LBP, 2) weekly messages, and 3) contact. The contents of the booklet and weekly messages were identical to those provided to the INT group. They also received a text message in week 4 to encourage them to use the app.
Sample size calculationInitially, we aimed for a sample size of 66 participants, expecting a significant difference of 1.5 pain intensity and 4 points in disability between groups. However, due to time constraints, we recruited only 47 participants before the deadline. Additionally, the specific nature of PSW, who often work in shifts, posed significant challenges. These irregular schedules limited their availability and willingness to participate, complicating recruitment further. We conducted a post hoc power analysis, which indicated a statistical power of approximately 63 % based on the observed effect size (0.68) and a significance level of 5 %. Though this power is lower than planned, it reflects the challenges of recruiting within our specific population of interest.
Signal processingThe MVIC and sEMG signals were collected and synchronized through a program implemented on the MioGraph platform (Miotec, Brazil). The full signal processing is described in the Supplementary Material (Supplementary material - Fig. 5).
Statistical analysesWe employed descriptive statistics to outline participants' baseline characteristics. Between-group differences and 95 % confidence intervals (95 % CI) for post-treatment outcomes at eight and 16 weeks were assessed using repeated-measures linear mixed models, considering participants and time as random factors. Analyses adhered to intention-to-treat principles, with missing data addressed through multiple imputation techniques. Statistical significance was set at two-sided p-values <0.05. All statistical procedures were conducted using SPSS version 20.0 for Windows (IBM corporation, Somers, New York, USA).
ResultsA total of 47 PSW were randomized (INT: n = 23; CON: n = 24) (Fig. 1). Overall, 91.3 % (INT: n = 21) and 95.8 % (CON: n = 23) of participants completed the self-reported assessments, 82.6 % (INT: n = 19) and 83.3 % (CON: n = 20) of participants completed neuromuscular assessments after the eight week intervention, and 82.6 % (INT: n = 19) and 95.8 % (CON: n = 23) of participants completed self-reported assessments at the 16-week follow-up.
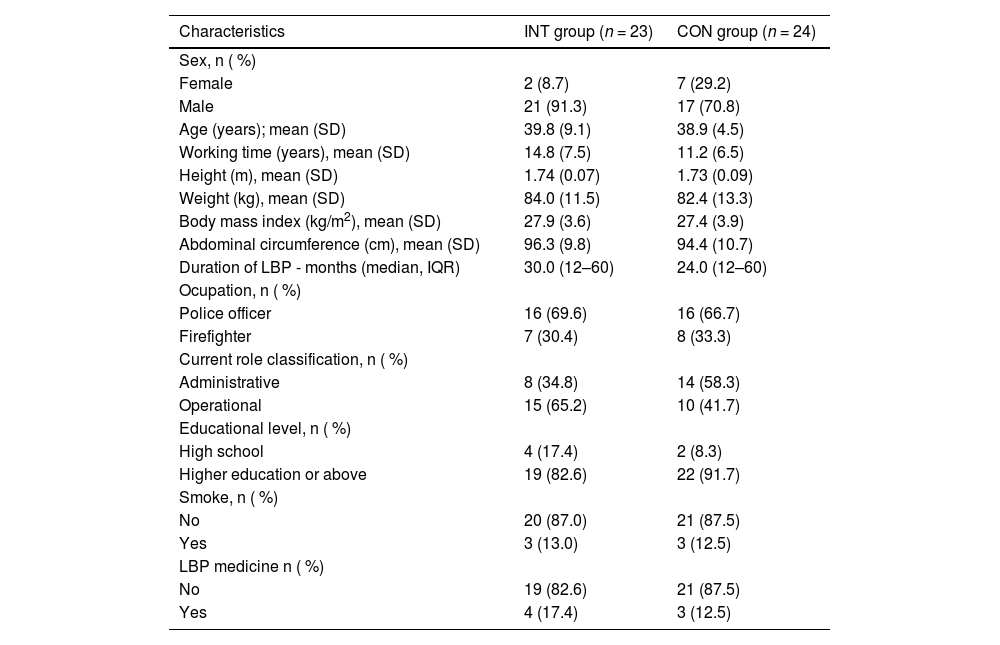
The participant's characteristics at baseline are presented in Table 1.
Characteristics of the participants at baseline.
CON, control group; INT, intervention group; LBP, low back pain.
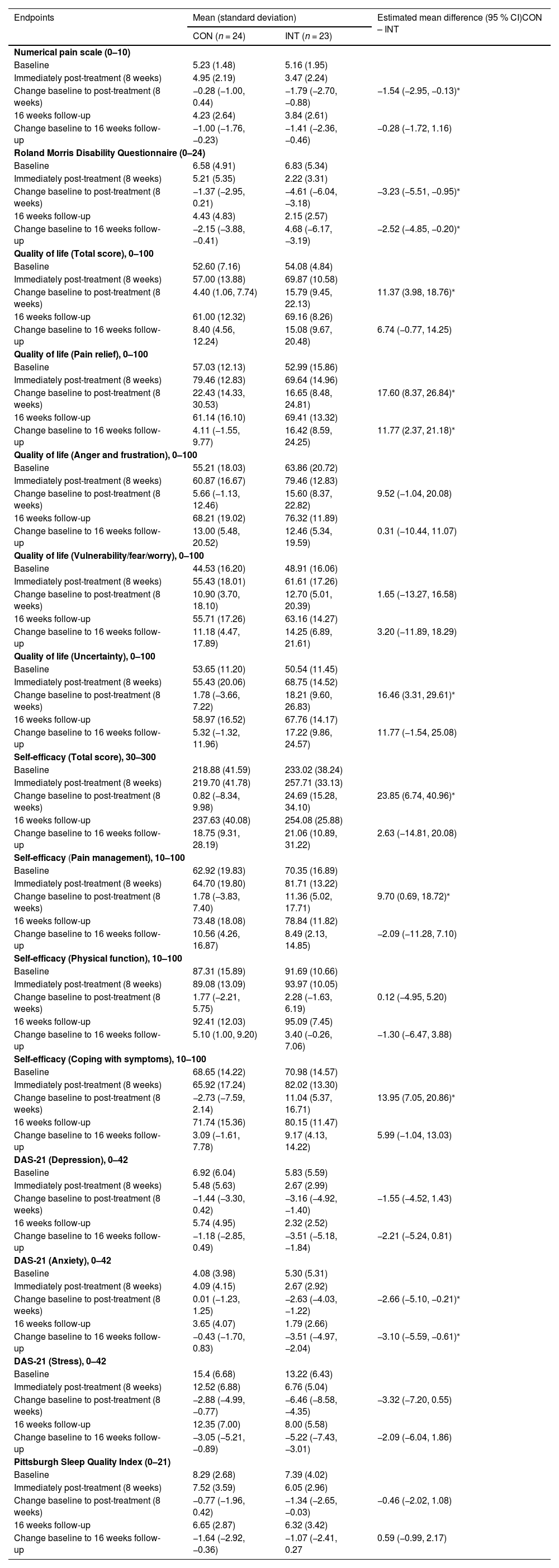
Those in the INT group had a greater decrease in pain intensity (MD = −1.54; 95 % CI: −2.95, −0.13) and disability (MD = −3.23; 95 % CI: −5.51, −0.95) than those in the CON group at eight weeks (Table 2). At the 16-week follow-up, those in the INT group had a greater decrease in disability (MD = −2.52; 95 % CI: −4.85, −0.20) than those in the CON group. There was no difference between groups in the decrease in pain intensity at 16 weeks (MD = −0.28; 95 % CI: −1.72, 1.16).
Between-group comparisons for each self-reported numeric outcome, including primary (pain intensity and disability) and secondary outcomes (quality of life, self-efficacy, depression, anxiety and stress symptoms, and sleep quality).
| Endpoints | Mean (standard deviation) | Estimated mean difference (95 % CI)CON – INT | |
|---|---|---|---|
| CON (n = 24) | INT (n = 23) | ||
| Numerical pain scale (0–10) | |||
| Baseline | 5.23 (1.48) | 5.16 (1.95) | |
| Immediately post-treatment (8 weeks) | 4.95 (2.19) | 3.47 (2.24) | |
| Change baseline to post-treatment (8 weeks) | −0.28 (−1.00, 0.44) | −1.79 (−2.70, −0.88) | −1.54 (−2.95, −0.13)⁎ |
| 16 weeks follow-up | 4.23 (2.64) | 3.84 (2.61) | |
| Change baseline to 16 weeks follow-up | −1.00 (−1.76, −0.23) | −1.41 (−2.36, −0.46) | −0.28 (−1.72, 1.16) |
| Roland Morris Disability Questionnaire (0–24) | |||
| Baseline | 6.58 (4.91) | 6.83 (5.34) | |
| Immediately post-treatment (8 weeks) | 5.21 (5.35) | 2.22 (3.31) | |
| Change baseline to post-treatment (8 weeks) | −1.37 (−2.95, 0.21) | −4.61 (−6.04, −3.18) | −3.23 (−5.51, −0.95)⁎ |
| 16 weeks follow-up | 4.43 (4.83) | 2.15 (2.57) | |
| Change baseline to 16 weeks follow-up | −2.15 (−3.88, −0.41) | 4.68 (−6.17, −3.19) | −2.52 (−4.85, −0.20)⁎ |
| Quality of life (Total score), 0–100 | |||
| Baseline | 52.60 (7.16) | 54.08 (4.84) | |
| Immediately post-treatment (8 weeks) | 57.00 (13.88) | 69.87 (10.58) | |
| Change baseline to post-treatment (8 weeks) | 4.40 (1.06, 7.74) | 15.79 (9.45, 22.13) | 11.37 (3.98, 18.76)⁎ |
| 16 weeks follow-up | 61.00 (12.32) | 69.16 (8.26) | |
| Change baseline to 16 weeks follow-up | 8.40 (4.56, 12.24) | 15.08 (9.67, 20.48) | 6.74 (−0.77, 14.25) |
| Quality of life (Pain relief), 0–100 | |||
| Baseline | 57.03 (12.13) | 52.99 (15.86) | |
| Immediately post-treatment (8 weeks) | 79.46 (12.83) | 69.64 (14.96) | |
| Change baseline to post-treatment (8 weeks) | 22.43 (14.33, 30.53) | 16.65 (8.48, 24.81) | 17.60 (8.37, 26.84)⁎ |
| 16 weeks follow-up | 61.14 (16.10) | 69.41 (13.32) | |
| Change baseline to 16 weeks follow-up | 4.11 (−1.55, 9.77) | 16.42 (8.59, 24.25) | 11.77 (2.37, 21.18)⁎ |
| Quality of life (Anger and frustration), 0–100 | |||
| Baseline | 55.21 (18.03) | 63.86 (20.72) | |
| Immediately post-treatment (8 weeks) | 60.87 (16.67) | 79.46 (12.83) | |
| Change baseline to post-treatment (8 weeks) | 5.66 (−1.13, 12.46) | 15.60 (8.37, 22.82) | 9.52 (−1.04, 20.08) |
| 16 weeks follow-up | 68.21 (19.02) | 76.32 (11.89) | |
| Change baseline to 16 weeks follow-up | 13.00 (5.48, 20.52) | 12.46 (5.34, 19.59) | 0.31 (−10.44, 11.07) |
| Quality of life (Vulnerability/fear/worry), 0–100 | |||
| Baseline | 44.53 (16.20) | 48.91 (16.06) | |
| Immediately post-treatment (8 weeks) | 55.43 (18.01) | 61.61 (17.26) | |
| Change baseline to post-treatment (8 weeks) | 10.90 (3.70, 18.10) | 12.70 (5.01, 20.39) | 1.65 (−13.27, 16.58) |
| 16 weeks follow-up | 55.71 (17.26) | 63.16 (14.27) | |
| Change baseline to 16 weeks follow-up | 11.18 (4.47, 17.89) | 14.25 (6.89, 21.61) | 3.20 (−11.89, 18.29) |
| Quality of life (Uncertainty), 0–100 | |||
| Baseline | 53.65 (11.20) | 50.54 (11.45) | |
| Immediately post-treatment (8 weeks) | 55.43 (20.06) | 68.75 (14.52) | |
| Change baseline to post-treatment (8 weeks) | 1.78 (−3.66, 7.22) | 18.21 (9.60, 26.83) | 16.46 (3.31, 29.61)⁎ |
| 16 weeks follow-up | 58.97 (16.52) | 67.76 (14.17) | |
| Change baseline to 16 weeks follow-up | 5.32 (−1.32, 11.96) | 17.22 (9.86, 24.57) | 11.77 (−1.54, 25.08) |
| Self-efficacy (Total score), 30–300 | |||
| Baseline | 218.88 (41.59) | 233.02 (38.24) | |
| Immediately post-treatment (8 weeks) | 219.70 (41.78) | 257.71 (33.13) | |
| Change baseline to post-treatment (8 weeks) | 0.82 (−8.34, 9.98) | 24.69 (15.28, 34.10) | 23.85 (6.74, 40.96)⁎ |
| 16 weeks follow-up | 237.63 (40.08) | 254.08 (25.88) | |
| Change baseline to 16 weeks follow-up | 18.75 (9.31, 28.19) | 21.06 (10.89, 31.22) | 2.63 (−14.81, 20.08) |
| Self-efficacy (Pain management), 10–100 | |||
| Baseline | 62.92 (19.83) | 70.35 (16.89) | |
| Immediately post-treatment (8 weeks) | 64.70 (19.80) | 81.71 (13.22) | |
| Change baseline to post-treatment (8 weeks) | 1.78 (−3.83, 7.40) | 11.36 (5.02, 17.71) | 9.70 (0.69, 18.72)⁎ |
| 16 weeks follow-up | 73.48 (18.08) | 78.84 (11.82) | |
| Change baseline to 16 weeks follow-up | 10.56 (4.26, 16.87) | 8.49 (2.13, 14.85) | −2.09 (−11.28, 7.10) |
| Self-efficacy (Physical function), 10–100 | |||
| Baseline | 87.31 (15.89) | 91.69 (10.66) | |
| Immediately post-treatment (8 weeks) | 89.08 (13.09) | 93.97 (10.05) | |
| Change baseline to post-treatment (8 weeks) | 1.77 (−2.21, 5.75) | 2.28 (−1.63, 6.19) | 0.12 (−4.95, 5.20) |
| 16 weeks follow-up | 92.41 (12.03) | 95.09 (7.45) | |
| Change baseline to 16 weeks follow-up | 5.10 (1.00, 9.20) | 3.40 (−0.26, 7.06) | −1.30 (−6.47, 3.88) |
| Self-efficacy (Coping with symptoms), 10–100 | |||
| Baseline | 68.65 (14.22) | 70.98 (14.57) | |
| Immediately post-treatment (8 weeks) | 65.92 (17.24) | 82.02 (13.30) | |
| Change baseline to post-treatment (8 weeks) | −2.73 (−7.59, 2.14) | 11.04 (5.37, 16.71) | 13.95 (7.05, 20.86)⁎ |
| 16 weeks follow-up | 71.74 (15.36) | 80.15 (11.47) | |
| Change baseline to 16 weeks follow-up | 3.09 (−1.61, 7.78) | 9.17 (4.13, 14.22) | 5.99 (−1.04, 13.03) |
| DAS-21 (Depression), 0–42 | |||
| Baseline | 6.92 (6.04) | 5.83 (5.59) | |
| Immediately post-treatment (8 weeks) | 5.48 (5.63) | 2.67 (2.99) | |
| Change baseline to post-treatment (8 weeks) | −1.44 (−3.30, 0.42) | −3.16 (−4.92, −1.40) | −1.55 (−4.52, 1.43) |
| 16 weeks follow-up | 5.74 (4.95) | 2.32 (2.52) | |
| Change baseline to 16 weeks follow-up | −1.18 (−2.85, 0.49) | −3.51 (−5.18, −1.84) | −2.21 (−5.24, 0.81) |
| DAS-21 (Anxiety), 0–42 | |||
| Baseline | 4.08 (3.98) | 5.30 (5.31) | |
| Immediately post-treatment (8 weeks) | 4.09 (4.15) | 2.67 (2.92) | |
| Change baseline to post-treatment (8 weeks) | 0.01 (−1.23, 1.25) | −2.63 (−4.03, −1.22) | −2.66 (−5.10, −0.21)⁎ |
| 16 weeks follow-up | 3.65 (4.07) | 1.79 (2.66) | |
| Change baseline to 16 weeks follow-up | −0.43 (−1.70, 0.83) | −3.51 (−4.97, −2.04) | −3.10 (−5.59, −0.61)⁎ |
| DAS-21 (Stress), 0–42 | |||
| Baseline | 15.4 (6.68) | 13.22 (6.43) | |
| Immediately post-treatment (8 weeks) | 12.52 (6.88) | 6.76 (5.04) | |
| Change baseline to post-treatment (8 weeks) | −2.88 (−4.99, −0.77) | −6.46 (−8.58, −4.35) | −3.32 (−7.20, 0.55) |
| 16 weeks follow-up | 12.35 (7.00) | 8.00 (5.58) | |
| Change baseline to 16 weeks follow-up | −3.05 (−5.21, −0.89) | −5.22 (−7.43, −3.01) | −2.09 (−6.04, 1.86) |
| Pittsburgh Sleep Quality Index (0–21) | |||
| Baseline | 8.29 (2.68) | 7.39 (4.02) | |
| Immediately post-treatment (8 weeks) | 7.52 (3.59) | 6.05 (2.96) | |
| Change baseline to post-treatment (8 weeks) | −0.77 (−1.96, 0.42) | −1.34 (−2.65, −0.03) | −0.46 (−2.02, 1.08) |
| 16 weeks follow-up | 6.65 (2.87) | 6.32 (3.42) | |
| Change baseline to 16 weeks follow-up | −1.64 (−2.92, −0.36) | −1.07 (−2.41, 0.27 | 0.59 (−0.99, 2.17) |
CON, control group; INT, intervention group.
At 8 weeks, participants in the INT group had greater improvements than those in the CON group in total score of quality of life (MD = 11.37; 95 % CI: 3.98, 18.76), pain relief (MD = 17.60; 95 % CI: 8.37, 26.84) and uncertainty (MD = 16.46; 95 % CI: 3.31, 29.61). No between-group differences were observed in other domains at 8 weeks. At 16 weeks, the INT group showed greater improvement than the CON group only in the pain relief domain (MD =11.77; 95 % CI: 2.37 to 21.18), with no differences in change between groups in the remaining domains (Table 2).
Self- efficacyAt 8 weeks, the INT group demonstrated greater improvement in total self-efficacy (MD = 23.85; 95 % CI: 6.74, 40.96), pain management (MD = 9.70; 95 % CI: 0.69, 18.72), and coping with symptoms (MD = 13.95; 95 % CI: 7.05, 20.86) compared to the CON group. No difference between groups was observed in physical function-related self-efficacy at this time point. At 16 weeks, there were no significant differences between groups in the change in any self-efficacy domains (Table 2).
Depression, anxiety, and stress symptomsAt 8 weeks, the INT group showed greater reductions in anxiety symptoms than the CON group (MD = −2.66; 95 % CI: −5.10, −0.21). This difference persisted at 16 weeks (MD = −3.10; 95 % CI: −5.59, −0.610). No between-group differences were observed for changes in depression or stress symptoms at either 8 or 16 weeks (Table 2).
Sleep qualityThere were no significant differences between the INT and CON groups in the change in sleep quality at either 8 weeks (MD = −0.46; 95 % CI: −2.02, 1.08) or 16 weeks (MD = 0.59; 95 % CI: −0.99, 2.17) (Table 2).
Work abilityRegarding work ability, a consistent reduction in the percentages of work disability was observed for the participants in the INT group throughout the study. At baseline, 17.4 % of participants reported working in less physically demanding roles due to low back and/or leg pain, but this number decreased to 14.3 % after 8 weeks and to 10.5 % after 16 weeks of intervention. In comparison, the CON group, at baseline, had 12.5 % of participants reporting being in less demanding roles due to pain, and this number decreased to 8.7 % after 8 weeks, remaining stable at 8.7 % after 16 weeks.
Adverse eventsNo adverse events related to the interventions were reported.
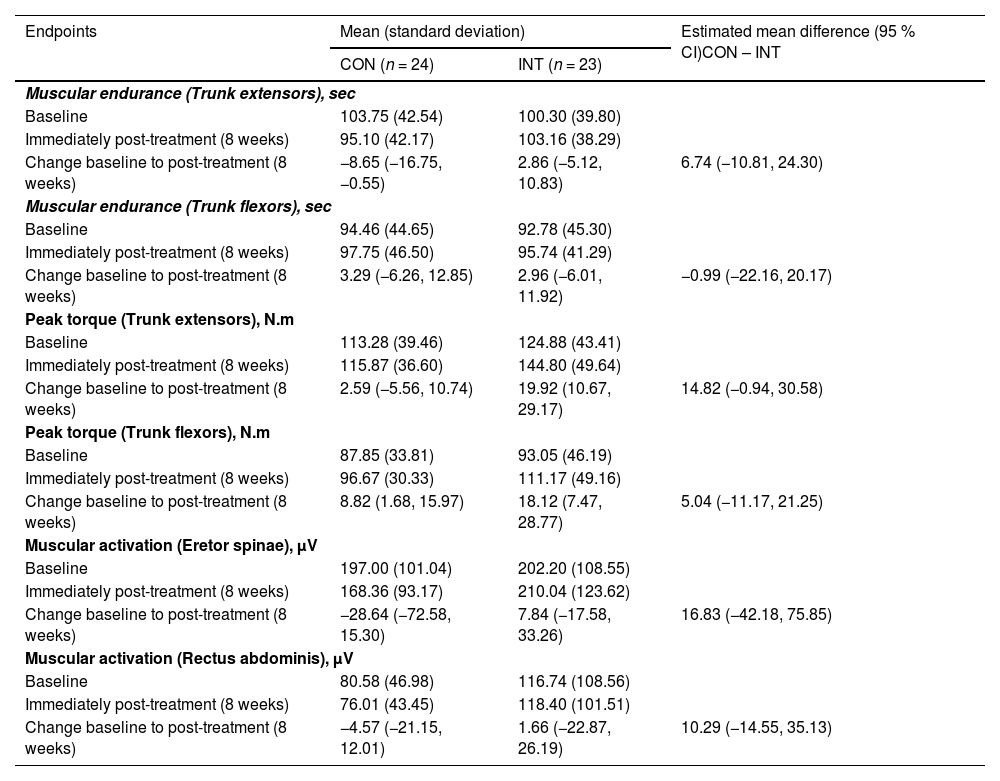
Neuromuscular outcomesNeuromuscular outcomes results are displayed in Table 3. No difference between groups was observed in muscular endurance, peak torque, and muscular activation of trunk extensors and flexors.
Between-group comparisons for each neuromuscular outcome, including muscular endurance, peak torque, and muscular activation.
CON, control group; INT, intervention group.
We revealed that participants in the INT group experienced greater improvements in pain intensity, disability, anxiety symptoms, self-efficacy, and quality of life compared to those in the CON group after 8 weeks of intervention. Although these effects persisted at the 16-week follow-up for disability, quality of life related to pain relief, and anxiety symptoms, some aspects warrant careful consideration.
Our results showed that the INT group had a greater reduction in pain (15.0 %) and disability (13.5 %) scores, as well as an improvement in quality of life (11.4 %−17.6 %), compared to the CON group at eight weeks, but with smaller mean effect sizes than those reported in other trials.41,42 However, others did not adopt intention-to-treat approaches and evaluated distinct populations (i.e., office workers and patients at a physical therapy clinic), making it difficult to compare the results. Although we found significant differences in pain intensity favoring the INT group, from the clinical perspective, this difference does not reach the minimal clinical difference expected to impact participant’s pain experience.43 However, from a public health perspective, even small changes in pain intensity can significantly impact workers’ functional performance.44 In addition, the intervention effects on disability at 16-week follow-up should be highlighted, indicating improved functionality that helps participants return to daily activities.
Despite these positive findings, the study's limited sample size and statistical power (63 %) must be acknowledged. While a larger sample might have increased the likelihood of detecting statistically significant differences, this would only be relevant if clinically meaningful effects were present. In our findings, several outcomes showed small between-group differences that were not statistically significant and also did not meet thresholds for clinical relevance, suggesting that increasing the sample size alone would likely not alter their clinical interpretation.
Evidence indicates that interventions incorporating physical exercises, particularly core strengthening, are more effective than usual treatments in alleviating pain and enhancing functional disability among patients with chronic LBP.15,45 However, we did not find differences between the groups in the neuromuscular outcomes of the trunk extensor and flexor muscles (strength, resistance, and muscle activity). In contrast to previous in-person interventions among firefighters with chronic LBP,46,47 we noted improvements in pain, disability, and quality of life after eight weeks, persisting at 16 weeks for disability and pain-related quality of life enhancement. Core strengthening exercises may alter deep trunk muscle behavior (e.g., transversus abdominis and lumbar multifidus) during functional tasks.48 Several mechanisms hypothesize how exercises can enhance deep trunk muscle coordination, such as reducing load and improving movement quality, thereby ameliorating pain-related outcomes.49 These findings are pertinent for PSW, who frequently handle trunk loads (∼20 kg), altering spine biomechanics and leading to forward trunk inclination, which overloads hip extensor muscles50 and exacerbates pain levels.
Improvements in anxiety symptoms were significantly greater in the INT group compared to the CON group at both follow-up time points, reinforcing the potential of exercise-based m-health interventions to positively influence mental health outcomes in PSW. While Almhdawi et al.42 found no differences in anxiety symptoms between exercise (intervention) and message-only (control) groups delivered via the app over six weeks, Zheng et al.51 reported improvements in depression and anxiety symptoms among patients with chronic LBP using m-health-based exercise (guidance plus education) compared to exercise-only app users after six weeks. Discrepancies in interventions, comparators, and duration may account for these variations. Our findings suggest significant mental health benefits for PSW, potentially enhancing work performance, underscoring the importance of comprehensive treatment approaches addressing their multifaceted needs.52
Prior research indicates that high self-efficacy correlates with positive outcomes across various life domains, including chronic pain management, quality of life, and effective utilization of m-health interventions, aligning with our findings.53 Sandal et al.54 observed between-group differences in pain-related self-efficacy among patients with LBP using a self-managed, artificial intelligence-based app alongside usual care compared to those receiving usual care alone at three months. While these results support ours, differences in sample characteristics, LBP symptoms, and intervention apps should be noted.54 In the context of chronic LBP among PSW, a strong belief in their ability to manage pain and fulfill their responsibilities can serve as a potent coping mechanism for addressing associated physical and emotional challenges.
Poor quality of sleep constitutes a potential risk factor for experiencing LBP,9 and is a substantial concern among PSW.55,56 Although face-to-face approaches to exercise treatments improve sleep quality in some populations,57,58 we did not find significant improvements in sleep quality in the INT group compared to the CON group, corroborating previous studies with office workers.42 However, the complexity of the conditions faced by PSW may require more comprehensive and adapted approaches.
Our findings complement the guidelines on managing chronic LBP, which present non-pharmacological treatments, such as exercises or pain education, among the main pillars to reduce pain and disability related to chronic LBP.59–61 Furthermore, recent meta-analyses showed that digital support systems can benefit chronic LBP self-management.62–64 In this context, our results contribute to the evidence on telerehabilitation, mainly in PSW with chronic LBP, whose access to health care is often limited.
The "MY SAFE BACK" app was developed based on self-management concepts for treating chronic LBP, incorporating practical exercises and health education. This approach aims to address geographical barriers faced by PSW, expanding access to treatment. It also helps overcome stigmas associated with seeking conventional healthcare among PSW, who may be concerned about how colleagues or superiors perceive their actions.65,66 Moreover, PSW irregular shifts and long hours make face-to-face treatments challenging, making an app-based program a flexible solution. Lastly, the app's self-management capability empowers PSW to actively manage their chronic LBP, improving symptoms and promoting greater health control.
LimitationsThe limitations of our study must be acknowledged. First, participants were not blinded to the intervention. Second, there was an initial imbalance in group allocation. However, we adjusted by conducting a new randomization (block randomization) as reported in the sample size calculation section. The need for a smartphone with an Android operating system can also be considered a limitation. Furthermore, the failure to achieve the calculated sample size represents a limitation or deviation from the study protocol, potentially affecting the statistical power of our analyses. Further research is required to determine the cost-effectiveness and long-term benefits (beyond 12 months) of the "MY SAFE BACK" app.
ConclusionA m-health-based core stability exercise self-management program combined with health education was more effective in reducing pain intensity, disability, and anxiety symptoms and improving quality of life and self-efficacy than m-health-based health education alone for managing chronic LBP in PSW. Follow-up effects of the proposed approach were observed for disability, quality of life, and anxiety symptoms. There was no difference in sleep quality, depression symptoms, stress, and neuromuscular outcomes between interventions, and the treatment effect was maintained in the mid-term follow-ups for disability, anxiety, and quality of life related to pain relief.
Trial registration: The study was prospectively registered at ClinicalTrials.gov (NCT05481996. Registered on August 01, 2022).
The authors declare no competing interest.
We thank all the police officers and firefighters who participated in this study. Their important contributions made this study possible.
This study received financial support from Programa de Apoio à Pós-Graduação (PROAP) of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, for purchasing the materials made available to participants. Involved personnel has been founded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, grant number 315430/2021–4 (C.L.A), and financed in part by the CAPES, finance code 001 (E.L.C, B.B.V, V.H.G.P, and D.M.J). The funding source played no role in the study design, collection, analysis, and interpretation of the data, nor in the manuscript's writing and the decision to submit the manuscript for publication.