Respiratory muscle training has shown to increase strength of the respiratory muscles following a stroke. However, low duration and/or intensity of training may be responsible for the small effect size seen and/or absence of carry-over effects to an activity, e.g., walking. Therefore, an investigation of the effects of long-duration, high-intensity respiratory muscle training is warranted.
ObjectiveThis proposed protocol for a randomized clinical trial will examine the efficacy of high-intensity respiratory muscle training to increase strength and improve activity following a stroke.
MethodsThis study will be a two-arm, prospectively registered, randomized controlled trial, with blinded assessors. Thirty-eight individuals who have suffered a stroke will participate. The experimental group will undertake a 40-min of respiratory muscle training program, seven days/week, for eight weeks in their homes. Training loads will be increased weekly. The control group will undertake a sham respiratory muscle training program with equivalent duration and scheduling of training. The primary outcome will be the strength of the inspiratory muscles, measured as maximal inspiratory pressure. Secondary outcomes will include expiratory muscle strength, inspiratory muscle endurance, dyspnea, respiratory complications, and walking capacity. Outcomes will be collected by a researcher blinded to group allocation at baseline (Week 0), after intervention (Week 8), and one month beyond intervention (Week 12).
ConclusionHigh-intensity respiratory muscle training may have the potential to optimize the strength of the respiratory muscles following a stroke. If benefits are carried over to activity, the findings may have broader implications, since walking capacity has been shown to predict physical activity and community participation on this population.
Following a stroke, the loss of the ability to generate normal amounts of force also affects the respiratory muscles.1 Mean inspiratory muscle strength has been reported as ∼51cmH2O in stroke patients, compared with ∼92cmH2O in healthy adults, and expiratory strength as 67cmH2O, compared to ∼117cmH2O.2 That is, respiratory muscle strength in people suffering from a stroke is half of that expected in healthy adults. Weakness of the respiratory muscles has been related to decreased walking capacity,3,4 which, in turn, has been related to restrictions in community participation. Additionally, respiratory muscle weakness may limit participation in community exercise programs, due to the reduced capacity to walk long distances.5
Respiratory muscle training has the potential to increase the strength of the respiratory muscles,6 and its effect has been previously examined in people with acute and chronic respiratory conditions,7,8 heart failure,9 and stroke.1,10,11 With this type of training, patients are asked to perform repetitive breathing exercises against an external load (e.g., using a flow-dependent resistance device or a pressure threshold device?8,10), in order to increase muscle strength and/or endurance. There have been four systematic reviews1,10–12 with meta-analysis of randomized trials examining the effect of respiratory muscle training on measures of respiratory muscle strength following a stroke. However, two reviews included only two trials of inspiratory training with substantial statistical heterogeneity (I2=95%), leading to inconclusive findings.1,11 More recent systematic reviews10,12 examined the effect of both inspiratory and expiratory muscle training following a stroke. Gomes-Neto et al.12 included seven randomized trials of reasonable quality and reported increases of 7cmH2O in inspiratory strength (95%CI: 3–12; I2=45%) and forced vital capacity (MD 2L, 95%CI: 1–3, I2=86%), forced expiratory volume at 1 second (MD 1.2L, 95%CI: 1–2; I2=51%), and exercise tolerance (SMD 0.7, 95%CI: 0.2–1.2; I2=0). However, the results were not significant for expiratory strength (MD 6cmH2O, 95%CI: −4 to 15; I2=57%). Menezes et al.10 included five randomized trials of reasonable quality and reported increases of 7cmH2O in inspiratory strength (95%CI: 1–14; I2=33%) and 13cmH2O for expiratory strength (95%CI: 1–25; I2=12%). However, the results were inconclusive regarding inspiratory endurance, occurrence of respiratory complications, and carry-over to everyday activity. Furthermore, only one of the trials included combined training (i.e., inspiratory plus expiratory training), a combination which has the potential to optimize the effects of training.
Although evidence appears to recommend respiratory muscle training to increase the strength of the respiratory muscles, the magnitude of the effect was small and the effect size may potentially be increased if training is of appropriate duration and/or intensity. The majority of the trials, which were included in the reviews, targeted only inspiratory or expiratory muscles, had a mean training duration of four weeks, and did not systematically progress training. As respiratory muscles respond to training stimuli in a similar fashion to other skeletal muscles, they can be overloaded by requiring them to work longer, at high intensities, and/or more frequently, than they are normally accustomed to.2 Thus, the effect of respiratory muscle training on strength and activity following a stroke may be increased with higher intensity training (i.e., increased frequency, duration and training of both muscle groups).
Therefore, this protocol for a randomized clinical trial will examine whether high-intensity respiratory muscle training is effective in increasing strength and endurance of the respiratory muscles, decreasing dyspnea sensation and respiratory complications, and improving walking capacity following a stroke.
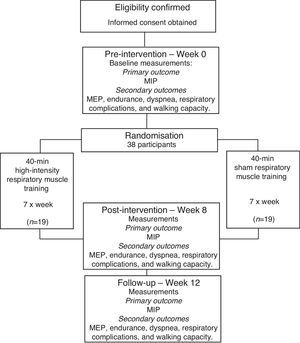
MethodsStudy designA prospective, randomized trial with concealed allocation, blinded assessors, and intention-to-treat analysis will be carried-out (Fig. 1). Community-dwelling people who have suffered a stroke will be recruited and randomly allocated to either a respiratory muscle training (experimental) group or a sham training (control) group. Outcome measures will be collected by trained researchers at baseline (Week 0), at the end (Week 8), and one month after the cessation of the training (Week 12). Data collection and analysis will be carried-out by a researcher blinded to the group allocation. Participants will be informed about the study and will provide consent before participating in the trial. The study obtained ethical approval from the Institutional Research Ethical Committee (CAAE: 40290114.8.0000.5149) of the Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. The trial was prospectively registered at the ClinicalTrials.gov (NCT02400138).
Patient population – inclusion and exclusion criteriaParticipants will be people who have suffered a stroke, who will be eligible if they:
- •
are >3 months and <5 years after their last stroke episode;
- •
are >20 years of age;
- •
have maximal inspiratory pressure <80cmH2O or maximal expiratory pressure <90cmH2O13;
- •
have not undertaking any respiratory training;
- •
have provided written consent.
Participants will be excluded if they have:
- •
cognitive deficits, which might prevent them from following instructions;
- •
facial palsy, associated respiratory diseases, or unstable conditions, which might prevent testing or training;
- •
undergone thoracic or abdominal surgery.
Physical therapists, who will deliver both interventions (experimental and control), will be eligible to work with the participants if they have at least two years of professional experience in the area of neurological or pulmonary rehabilitation.
RandomizationThe randomization method will be computer-generated, independent, using random permutation blocks of four participants, and stratified according the maximal inspiratory pressure (≥45cmH2O – respiratory muscle weakness; <45cmH2O – severe respiratory muscle weakness), to ensure an even spread between the groups. The participant allocation will be concealed in sequentially numbered and sealed opaque envelopes, prepared prior to the study by a research assistant, who will not be involved in the study. After the baseline measures have been collected, participants will be randomly allocated to the experimental or control groups by the intervention therapist, using the content in the sealed opaque envelopes.
InterventionThe respiratory muscle training will be carried-out using an Orygen Dual Valve device (Forumed S.L., Barcelona, CAT, ESP). This respiratory device allows patients to train the inspiratory and expiratory muscles simultaneously and to adjust the loads independently. The Orygen Dual Valve is a relatively cheap, portable, easy to use piece of equipment and provides workloads up to 70cmH2O with load adjustments of 10cmH2O.14
The experimental group will receive home-based respiratory muscle training, totaling 40min per day, seven days per week, over eight weeks. The 40-min daily training will be split into two daily sessions (i.e., morning and afternoon). Each daily session will be comprised of 4 four-minute sets of respiratory training with one-minute rest intervals between each set. The resistance will be provided by the Orygen Dual Valve, which allows individuals to exercise the inspiratory and expiratory muscles simultaneously during the training session.15 Training will be individually tailored for each participant. The initial training load for each participant will be set at 50% of his/her maximal baseline inspiratory and expiratory strength for both inspiratory and expiratory strength training. The participants will be trained and instructed to do the exercise program on their own with no supervision. Once a week, during the physical therapist's home visit, the clinician will determine the new values for maximal inspiratory and expiratory strength, and will adjust the load to 50% of the new values. The device will be covered with opaque material, so that participants will be blinded to the training load.
The control group will receive a sham intervention. Sham respiratory muscle training will be delivered using a Orygen Dual Valve with no resistance (0cmH2O) or progression, over the 8 week period, 40min per day, seven days per week. The device will be covered with opaque material, so that participants will be blinded to the fact that they are receiving no training load. The control group will receive the same training and testing schedule as the experimental group. This will avoid bias related to amount of attention given to participants. If training proves to be effective, the control group may receive the experimental training program after the experiment is complete and if it proves to be effective.
The respiratory muscle training will be undertaken in the participant's home environment, which will reduce the cost of the intervention and may result in good compliance.16 On the other hand, this means that the training will not be directly supervised. To record the compliance, participants will receive a diary to register the time and days of each training session and their daily training volume. When required, a caregiver will be instructed how to help them. To encourage the participants to comply with the protocol, both groups will be asked to sign a contract of commitment to the training program. All the participants will be evaluated and receive all the information regarding the home-based training in a research laboratory.
Primary outcomeThe primary outcome to be measured is strength of the inspiratory muscles (i.e., maximal inspiratory pressure generated during maximum resistance of inspiration, measured using a digital manovacuometer17), and reported as pressure (cmH2O), following the recommended guidelines for the use of the manovacuometer.18
Secondary outcomesSecondary outcomes to be measured are the strength of the expiratory muscles, inspiratory endurance, dyspnea, respiratory complications, and walking capacity.
Strength of the expiratory muscles (i.e., maximal expiratory pressures generated during maximum resistance of expiration), will be measured using a digital manovacuometer,17 and reported as pressure (cmH2O), following the recommended procedures for the use of the manovacuometer.18
Inspiratory endurance will be measured using a flow-resistive loading device (POWERbreathe KH1) and reported as the number of breaths. Participants will be asked to breathe against a sub-maximal inspiratory load (i.e., 50% of their maximal inspiratory pressure measured at baseline, until task failure), following the recommended guidelines for the flow-resistive loading device.19
Dyspnea will be measured using the Medical Research Council Scale, which is a 5-point scale, in which 0 indicates ‘breathless only with strenuous exercise’ and 4 indicates ‘too breathless to leave the house’.20
The incidence of respiratory complications will be measured weekly during the 12 weeks of the study by asking the participants whether they were admitted to a hospital and how often for respiratory causes (e.g., pneumonia or lung infections).
Walking capacity will be measured using the 6-min Walk Test and reported as distance (m) covered in 6min. Using a standardized protocol for the 6-min Walk Test,21 participants will be instructed to cover the maximum distance possible in 6min, taking rests as needed.
Data monitoringAn independent researcher, who will be blind to the group allocations, will monitor any adverse effects and perform database management and statistical analyses. The treating physical therapists will be responsible for the monitoring of exercise doses and participants program compliance.
Sample size estimatesThirty-eight participants will be recruited, using strength of inspiratory muscles as the primary outcome. The sample size was calculated to reliably detect a between-group difference of 15cmH2O, with 80% power, at a two-tailed significance level of 0.05, and an expected drop-out rate of 15%. In a randomized trial with a similar population,22 the strength of the inspiratory muscles was 57cmH2O (SD 15) using the same measurement procedures. The least number of participants needed to detect a 15cmH2O difference between two independent groups in that study was 16 subjects per group. Based on the assumption that about 15% of the participants might drop-out during the course of the study, a target of 38 participants in total is set.
Statistical analysesData collection will return six variables, which reflect impairments and activity limitations: inspiratory and expiratory strength (cmH2O), inspiratory endurance (i.e., number of breaths), dyspnea (Medical Research Council scale 0–4), respiratory complications (i.e., number of hospital admissions), and walking capacity (i.e., meters in 6min). There are two factors (group×time), with repeated measures on the time factor. Two-way analyses of variance with repeated measures at all time-points for all outcomes will be reported to evaluate the statistical significance of between-group differences. The mean between-group difference and 95% confidence intervals will be reported for all outcomes. The effect of the intervention will be calculated based on intention-to-treat analyses.
DiscussionSignificance of the studyThe proposed clinical trial will examine the efficacy of a high-intensity respiratory muscle training protocol delivered to people following a stroke. Although previous systematic reviews have shown positive effects of respiratory muscle training on strength of the respiratory muscles, the effect sizes were too small and insufficient to provide carry-over effects to everyday activities.10,12 In response to this challenge, a higher intensity training (i.e., increased frequency, duration, and targeting both inspiratory and expiratory muscles), which may result in relevant increases in respiratory muscle strength. Since respiratory muscle training not only imposes a resistance to the respiratory muscles, but also causes hyperventilating for prolonged periods of time, it may have an additional effect on respiratory muscle endurance,2,23,24 which could translate into a more efficient use of the respiratory muscles in activities of daily living. Furthermore, if benefits are carried over to activity, the findings may have broader implications, since walking capacity has been shown to predict physical activity and community participation following a stroke.5
In addition, a previous systematic review10 has indicated that respiratory muscle training has the potential to decrease the risk of respiratory complications after stroke. It is well known that pneumonia and respiratory illness are the most common reasons associated with hospital admissions and death following a stroke, responding for 15% of the readmissions.25 The adoption of interventions capable of preventing the occurrence of respiratory complications may substantially improve the long-term outcomes of patients following a stroke.26 Our clinical trial aims to clarify the evidence regarding the effects of a respiratory muscle training on the occurrence of respiratory complications. If training benefits will lead to decreases number of readmissions/deaths due to respiratory complications, the findings may have broader implications, such as reduced costs arising from immediate and long-term health care, increased production due to reduced sick leave, and early retirements.
The experimental intervention will be delivered via the Orygen-Dual Valve®. This device is a relatively inexpensive (costs about 60 US dollars) and portable respiratory trainer that also allows patients to simultaneously work both the inspiratory and expiratory muscles.14 Although it has been recently developed, studies have proven its efficacy in patients with chronic heart failure14 and stroke.15 If the intervention provides better results than the sham treatment, this device could be a good option for patients with respiratory muscle weakness.
In conclusion, the results of the trial may result in an important advance in neurological/pulmonary rehabilitation. First, the magnitude of effect may be higher than previous trials, due to the increased intensity of training. Second, if benefits are carried over to activity, community participation may be enhanced, given that the 6-min Walk Test is a strong predictor of community walking in people following a stroke.27 Third, if the results lead to a reduction in hospital admissions, respiratory muscle training could help reducing direct and indirect costs associated with a stroke.
Strengthens and weakness of the proposed protocolThe strength of the proposed protocol is that it is a randomized controlled trial that has been prospectively registered. The trial also includes concealed allocation and an intention-to-treat approach. The sample size has been calculated to provide appropriate statistical power to detect between-group differences in the primary outcome. The assessor responsible for collecting the outcome data will be blind to group assignment. The physical therapist, who delivers the training, has clinical experience and a masters degree in neurological rehabilitation. This trial has some limitations, since participants and therapist cannot be really blinded, which is impractical during the delivery of complex interventions. The experimental and control interventions consist of home exercises, which depend on participants’ motivation. It is not possible to predict the amount of home exercises that will be performed. Strategies to encourage participants to comply with the protocol, such as contract of commitment to the training, phone calls, and weekly visits, are planned.
Conflicts of interestThe authors declare no conflicts of interest.
The trial is funded by the following national funding agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – grant number 304434/2014-0) and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG – PPM 00006-14). Data will be stored at the Universidade Federal de Minas Gerais, Brazil.
Trial registration: Clinical Trials, NCT02400138. Registered on March 23rd, 2015 (https://clinicaltrials.gov/show/NCT02400138).