Home-based pulmonary rehabilitation is a promising intervention that may help patients to overcome the barriers to undergoing pulmonary rehabilitation. However, home-based pulmonary rehabilitation has not yet been investigated in patients with bronchiectasis.
ObjectivesTo investigate the effects of home-based pulmonary rehabilitation in patients with bronchiectasis.
MethodsAn open-label, randomized controlled trial with 48 adult patients with bronchiectasis will be conducted. Interventions: The program will consist of three sessions weekly over a period of 8 weeks. Aerobic exercise will consist of stepping on a platform for 20min (intensity: 60–80% of the maximum stepping rate in incremental step test). Resistance training will be carried out using an elastic band for the following muscles: quadriceps, hamstrings, deltoids, and biceps brachii (load: 70% of maximum voluntary isometric contraction). Control: The patients will receive an educational manual and a recommendation to walk three times a week for 30min. All patients will receive a weekly phone call to answer questions and to guide the practice of physical activity. The home-based pulmonary rehabilitation group also will receive a home visit every 15 days. Main outcome measures: incremental shuttle walk test, quality of life, peripheral muscle strength, endurance shuttle walk test, incremental step test, dyspnea, and physical activity in daily life. The assessments will be undertaken at baseline, after the intervention, and 8 months after randomization.
DiscussionThe findings of this study will determine the clinical benefits of home-based pulmonary rehabilitation and will contribute to future guidelines for patients with bronchiectasis.
Trial registration:www.ClinicalTrials.gov (NCT02731482). https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S00060X6&selectaction=Edit&uid=U00028HR&ts=2&cx=1jbszg
Pulmonary rehabilitation (PR) is the most effective non-pharmacological therapy to reduce dyspnea, improve exercise tolerance, and enhance quality of life in patients with chronic pulmonary diseases.1 Despite strong evidence, over 95% of patients with chronic obstructive pulmonary disease (COPD) who could benefit from PR are not referred to this therapy.2,3 Of those who are referred to PR, 8% to 50% do not take up the referral and, among those who start the program, approximately 20% do not complete it.4,5 The causes for this low uptake and adherence are multifactorial and include the lack of specialized programs, particularly outside urban centers, the insufficient number of qualified professionals, difficulties with transportation and its costs, and the difficulty of reconciling work activities with rehabilitation.6–8 In this context, home-based PR (HBPR) may be an alternative option to overcome the barriers to attendance at center-based programs.
COPD is the most investigated lung disease both in terms of outpatient PR and HBPR. In patients with COPD, HBPR was first described in the mid-90s9 and since then, numerous studies have shown that, similar to outpatient rehabilitation, home-based programs improve quality of life, increase exercise tolerance, and improve dyspnea without serious side effects.10–22 Since 2010, the effects of HBPR have been extended to other chronic lung diseases, such as idiopathic pulmonary fibrosis,23,24 asthma,7,25 and tuberculosis,26with similar results to those described in patients with COPD. However, the effects of HBPR have not yet been investigated in patients with bronchiectasis. Bronchiectasis is a severe, progressive disease with high socioeconomic impact,27–29 which includes extrapulmonary manifestations such as reduced functional capacity and peripheral muscle endurance.30–32
Patients with bronchiectasis usually receive PR in an outpatient context,33–39 and as noted in other chronic lung diseases, it improves the patient's physical capacity and reduces dyspnea, fatigue, and the number of exacerbations.1 Therefore, HBPR could also be beneficial for patients with bronchiectasis who cannot access an outpatient rehabilitation program. This study will contribute to future guidelines regarding HBPR for patients with bronchiectasis. The aim of this clinical trial is to investigate the short- and long-term effects of HBPR on functional capacity, quality of life, and peripheral muscle strength in patients with bronchiectasis.
MethodsDesignThis is a prospectively registered, two-arm, open-label, randomized controlled trial.
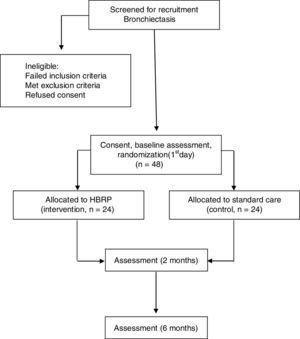
Recruitment and random selection of patientsParticipants will be recruited personally by the researchers from the Obstructive Disease Outpatient Clinic (Hospital das Clínicas of Universidade de São Paulo) and will be referred to the University Center for tertiary cardiopulmonary rehabilitation (Universidade Nove de Julho). The flow of participants through the study will follow the recommendations of the Consolidated Standards of Reporting Trials Statement and is outlined in Fig. 1. After written and verbal explanation regarding the objectives and procedures of the study, all participants will sign a consent form before starting the assessments. This study received approval from the Human Research Ethics Committee of Universidade Nove de Julho, São Paulo/SP, Brazil (no. 1249073), and the protocol was prospectively registered at www.ClinicalTrials.gov (NCT02731482).
The participants will be randomly assigned to receive either standard care (control group) or HBPR (intervention group). The randomization schedule will be generated using the website www.randomization.com. The randomization schedule will be concealed from participants and investigators by using consecutively numbered, sealed, and opaque envelopes.
Inclusion and exclusion criteriaThe study will include patients aged over 18 years with a clinical or tomographic diagnosis of bronchiectasis who are in a stable clinical state (absence of changes in the symptoms of dyspnea and in the volume and color of sputum). The exclusion criteria are smoking, primary diagnosis of another lung disease (asthma, COPD, interstitial lung disease, or cystic fibrosis), severe cardiovascular disease, inability to perform the tests and the training protocol due to musculoskeletal limitations, and significant levels of desaturation (pulse oxygen saturation ≤80%) during baseline exercise testing will be excluded.
ProvidersThe interventions, procedures, and evaluations will be carried out by a team of three physical therapists who are authors of this manuscript. They are part of the postgraduate program in rehabilitation sciences and have PhD and/or master's degree in pulmonary rehabilitation with at least 5 years of professional and academic experience.
Sample size calculationFor the primary outcome (distance walked in ISWT) based on a previous study35 we expect a difference between groups of 61.3m and a standard deviation of 63.2m. These estimates will result in an effect size of 0.83, based on an alpha error of 0.05, a beta error of 0.20, and allocation ratio of 1:1 (intervention and control). Therefore, the total sample size will be 40 individuals who will be randomly allocated to two groups (20 to the intervention group and 20 to the control group).
For the secondary outcome (distance walked in ESWT) based on a previous study,35 we expect a difference between groups of 307.8m and a standard deviation of 123.8m. These estimates will result in an effect size of 1.86, based on an alpha error of 0.05, a beta error of 0.20, and allocation ratio of 1:1 (intervention and control). Therefore, the total sample size will be 12 individuals who will be randomly allocated to two groups (6 to the intervention group and 6 to the control group).
For the secondary outcome of total score for quality of life on St. George's Respiratory Questionnaire based on a previous study,35 we expect a difference between groups of 6.6 points and a standard deviation of 5.4. These estimates will result in an effect size of 1.4 based on an alpha error of 0.05, a beta error of 0.20, and allocation ratio of 1:1 (intervention and control). Therefore, the sample size will be 20 individuals who will be randomly allocated to two groups (10 to the intervention group and 10 to the control group).
A sample size of 40 participants gives sufficient power to all outcomes, however we will add 20% due to possible non-completion, missing data, and dropouts. Therefore, 48 participants will be recruited. The sample size was calculated using the program G*Power 3 (Düsseldorf, Bundesland, Germany).
InterventionsHome-based pulmonary rehabilitation groupParticipants allocated to this group will take part in the home-based pulmonary rehabilitation protocol. HBPR could be beneficial for patients who cannot access an outpatient rehabilitation program for any reason. Studies with patients with other pulmonary chronic diseases show that HBPR is useful for improving quality of life, increasing exercise tolerance, and improving dyspnea without serious side effects,10–26 but the effects of HBPR have not yet been investigated in patients with bronchiectasis.
The program will consist of three non-supervised weekly sessions, each session lasting 50min on average, for eight weeks. After the initial evaluation, conducted at the Cardiopulmonary Rehabilitation Centre at Universidade Nove de Julho, participants will receive an educational booklet with information on the disease and an illustrated script of the exercise program.
Participants with a private smartphone will receive an MP3 file with the soundtrack that will dictate the intensity of aerobic training (see “stepping training and materials”). If they do not have a smartphone, a CD with the recorded sound file will be provided. The appropriate elastic band for resistance muscle training will also be provided (see “resistance training and materials”).
The first physical training session will be held in the Cardiopulmonary Rehabilitation Centre at Universidade Nove de Julho, where researchers will explain and guide the exercise routine. The participants in this group will carry out the following procedures:
HBPR proceduresThe HBPR procedures were designed to be easy to implement, easy to understand, and low in cost. The program is composed of four parts (warm-up/stretching, aerobic training, resistance training, and follow-up) as follows.40,41
Warm-up/stretchingThe warm-up will last approximately 5min and will consist of active upper- and lower-limb exercises. Stretching will also last about 5min and will include the pectoralis major, latissimus dorsi, trapezius, femoral quadriceps, and hamstrings muscles. Each stretch posture will be held for 30s.42
Aerobic training and materialsAerobic training will consist of stepping on a platform (20cm high, 60cm wide, 60cm long) that will be provided to the participant. The intensity of training will be set at a cadence corresponding to 60–80% of the maximum stepping cadence achieved on the incremental step test43 for a duration of 20min. During the training session, target training HR and dyspnea and fatigue will be established as a marker of training intensity. Dyspnea and/or fatigue should be maintained between 4 and 6 on the modified Borg scale.44 Target training HR should correspond on average to 70% of the predicted maximum HR, according to the following equation: HR=[(max−HR rest)]×0.7+HR rest.45 The HR will be measured using a HR monitor (Polar Precision Performance; Polar Electro, Kempele, Finland). If participants report a score for dyspnea and/or fatigue below 4 and/or target training HR below that established by the equation, the exercise intensity will be increased by increasing one level of the speed of stepping. One level of the exercise corresponds to an increment of two steps per minute above the previous level. The opposite will be done if the scores on the Borg scale and/or target training HR are above the set level. If the participants use oxygen therapy, exercises will be done with continuous oxygen flow, according to medical prescription. If substantial desaturation occurs during the aerobic training session (SpO2<80%), its intensity will be decreased in order to maintain the SpO2 above 80%.
Resistance training and materialsResistance training will last on average 20min. Exercises will be carried out with three sets of eight repetitions each, with 1min of rest between sets, using both limbs simultaneously and an elastic band (TheraBand®; The Hygenic Corporation, Akron, OH, USA).
The muscles to be trained will be quadriceps, hamstrings, deltoids, and biceps brachii. Table 1 shows the position and describes the movement that participants will make during resistance training. The load will be set at 70% of maximum voluntary isometric contraction measured using the dynamometer (model DLC/DN; Kratos, São Paulo, SP, Brazil) as shown in Table 2.46 Adjustments to the intensity of the exercise will be made by increasing the load of the elastic bands during scheduled home visits. Intensity will be guided by the modified Borg scale44 for the fatigue of trained muscle group. At the end of the three sets, participants should report a sensation of dyspnea and/or fatigue from 4 to 6. If the score is below 4, the load is increased by changing the elastic band to more resistant level; if the score reach values higher than 6, the load will be reduced.47
Description of muscle group, position, and movement for evaluation and peripheral muscle strength training.
| Muscle | Position | Starting point | Movement and range |
|---|---|---|---|
| Quadriceps | Sitting | Knee flexed at 90° | Legs extended to 180° |
| Hamstrings | Ventral decubitus | Legs extended (180°) | Knee flexion to 90° |
| Deltoid | Standing | Upper limbs alongside body; hands in neutral position | Shoulder abduction to 90° |
| Biceps | Standing | Upper limbs alongside body; hands in supine position | Elbow flexion to 120° |
Resistance (in kilograms) provided by the elastic bands according to color.
| Stretch (%) | Yellow | Red | Green | Blue | Black | Gray | Gold |
|---|---|---|---|---|---|---|---|
| 25 | 0.5 | 0.7 | 0.9 | 1.3 | 1.6 | 2.3 | 3.6 |
| 50 | 0.8 | 1.2 | 1.5 | 2.1 | 2.9 | 3.9 | 6.3 |
| 75 | 1.1 | 1.5 | 1.9 | 2.7 | 3.7 | 5.0 | 8.2 |
| 100 | 1.3 | 1.8 | 2.3 | 3.2 | 4.4 | 6.0 | 9.8 |
| 125 | 1.5 | 2.0 | 2.6 | 3.7 | 5.0 | 6.9 | 11.2 |
| 150 | 1.8 | 2.2 | 3.0 | 4.1 | 5.6 | 7.8 | 12.5 |
| 175 | 2.0 | 2.5 | 3.3 | 4.6 | 6.1 | 8.6 | 13.8 |
| 200 | 2.2 | 2.7 | 3.6 | 5.0 | 6.7 | 9.5 | 15.2 |
| 225 | 2.4 | 2.9 | 4.0 | 5.5 | 7.4 | 10.5 | 16.6 |
| 250 | 2.6 | 3.2 | 4.4 | 6.0 | 8.0 | 11.5 | 18.2 |
Source: Page et al.46
The participants will receive a weekly phone call from the researchers in order to answer any questions about the exercise protocol, to learn about the participant's condition, motivate him/her to execute the proposed training protocol, and verify and encourage adherence to the protocol. Every two weeks, the participant will receive a visit from a researcher at his/her home. During this visit, a supervised session of the program will take place in order to correct any errors made by the participant, demonstrate correct execution of the exercise, set the appropriate intensity of stepping exercise for aerobic training, and ensure progression of resistance bands for the training of peripheral muscle strength. Also, the diary will be collected and evaluated, adherence to the program will be analyzed, and questions from participants will be answered.
Control groupRecruitment and data will be collected from patients in intervention and controls in identical ways and at the same locations. Participants allocated to the control group will receive an educational booklet with the same content as the booklet given to participants in the training group. The guidelines on the physical exercises will be replaced by instructions on the performance of physical activities and walking at moderate intensity, three times a week for 30min. This information will also be given verbally by the researchers, but the participants will not receive any supervised physical training.
During the eight-week intervention, the participants will be contacted by telephone by the researchers each week to receive support and general advice, without discussing exercises or physical activity. The participants in this group will not receive home visits from researchers. At the end of the study (eight months after randomization), the participants in this group will be invited to take part in the HBPR program, identical to that of the experimental group. However, the data collected from this participation in HBPR program will not be part of the present study.
Outcome measures and blindingThe researchers will assess the two groups at baseline, immediately after the intervention (2 months), and eight months after the randomization (Fig. 1). Baseline data collection will include age, gender, body mass index, the FACED score,48 and pulmonary function.49,50
Primary outcome measures(a) Functional exercise capacityThe incremental shuttle walk test (ISWT) will be performed in a flat corridor 10m long. Two tests will be performed on the same day, with a 1-h rest period between them.51 HR and SpO2 will be monitored continuously (Nonim Medical Inc., Plymouth, MN, USA). Dyspnea and fatigue will be recorded at rest and immediately after the test using the Borg scales.44 The longest distance walked will be used for analysis. If the participant uses oxygen, the test will be done using continuous oxygen flow, following medical prescription.
Secondary outcome measures(a) HRQoLHRQoL will be assessed using Saint George's Respiratory Questionnaire.52,53 The questionnaire assesses three domains: symptoms, activities, and impact. The total and domain scores range from 0 to 100, with higher scores denoting poorer quality of life.
(b) Peripheral muscle strengthPeripheral muscle strength will be measured using a dynamometer (Kratos, São Paulo, SP, Brazil). The biceps brachii, deltoid, quadriceps femoris, and hamstring muscles will be evaluated during isometric contractions of elbow flexion, shoulder abduction, and leg extension and flexion, respectively. Three maximum isometric contractions will be performed on the dominant limb for each muscle group, with a 1-min rest period between contractions. The highest value will be considered in the analysis. If the last contraction differs by more than 5% from the previous contraction, the test will be performed again until this difference is less than 5%.54Table 1 offers a description of the strength measurement for each muscle group.
(c) Maximal exercise capacityThe incremental step test will be performed as previously described.55 The test pace is determined by sound signals, starting with 10 steps per minute and increasing constantly by two steps per minute. The participant will be encouraged to reach their maximum performance capacity, and the test will be ceased when the participant can no longer keep up with the pace dictated by beeps for 15s. HR and SpO2 will be measured every minute using a pulse oximeter (9500, Nonin, Plymouth, MN, USA). Dyspnea and fatigue will be measured by the modified Borg scale.44 If the participant uses oxygen therapy, the test will be performed with a constant oxygen flow, according to medical prescription. The main outcome will be number of steps. During the test, pulmonary gas exchange will be recorded (CPX Ultima, Medical Graphics Corporation, St. Paul, MN, USA) to obtain peak VO2.
(d) Exercise endurance capacityThe endurance shuttle walk test (ESWT) will be performed as recommended.51 In brief, the speed for ESWT will correspond to 85% of the peak oxygen uptake (VO2 peak) established by the following regression equation: VO2 peak=4.19+0.025×ISWT distance.56 The outcome will be the time to perform the test. HR, SpO2, arterial blood pressure, and modified Borg scale44 for dyspnea and fatigue will be recorded before and immediately at the end of the test. If the participant uses oxygen therapy, the test will be performed with constant oxygen flow, according to medical prescription.
(e) Physical activity in daily lifePhysical activity will be measured by a pedometer (Yamax PowerWalker, model PW-610, Tokyo, Japan). Participants will receive a pedometer and will be instructed to keep it in their pockets for five consecutive days during the week. However, the first and last day will discarded leaving the remaining three consecutive days for analysis.57
Participants will be instructed on how to set up the pedometer in the morning and use it throughout the day, removing it only to shower and sleep. The outcome will be the average number of steps per day.
(f) DyspneaThe modified Medical Research Council scale will evaluate the effect of breathlessness on daily activities.58 This scale is composed of five activities on which breathlessness is scored from 1 to 5, with higher scores denoting greater limitations to performance of activities of daily living.
BlindingDue to the nature of the interventions, it is not possible to blind patients and therapists, and due to lack of human resources, the outcomes of this trial will be collected by an unblinded outcome assessor.
Statistical analysisData will be entered into the Statistical Package for Social Sciences (SPSS, version 20.0, Chicago, IL, USA) for analysis. The Shapiro–Wilk test will be used to determine the normal or non-normal distribution of the data. Treatment-by-time interactions will be analyzed by two-way repeated-measures analysis of variance with Holm–Sidak post hoc test. The effect size will be calculated using Cohen's test. A p-value <0.05 will be considered statistically significant. All data will be analyzed with an intention to treat approach, with inclusion of all available data regardless of whether the intervention was completed.
DiscussionThis is the first study evaluating the effects of HBPR on exercise tolerance, peripheral muscle strength, quality of life, dyspnea, pulmonary function, and physical activity in daily life in patients with bronchiectasis. Following the principles of physical training used in HBPR for other chronic pulmonary disesases,7,9–26 we included a low-cost activity program that requires little space to be performed and is easy for the participant to understand.
Aerobic training will be based on the incremental step test, which can be easily done at home. We believe that this type of aerobic training in HBPR is more feasible than walking-based programs because it does not depend on participants having a physical space in which to train outside of their home, a common need in HBPR studies that use walking as a base of aerobic training. Moreover, regardless of weather conditions and the need for continuous oxygen therapy using a cylinder or concentrator, the participant performs the step training in their own home. Peripheral muscle strength training will be performed with elastic bands due to difficulties in transporting and storing weights and other exercise equipment in home environments with limited space. Elastic bands are a practical, low-cost option for strength training when there is no access to more expensive or sophisticated equipment and are as effective as other resources used in strength training.59 The major limitation of this trial will be the lack of blinding of the outcome assessor. This study will contribute to future guidelines on HBPR for patients with bronchiectasis.
Authors’ contributionAnderson José: conception and design, data acquisition, data analysis and interpretation, drafting the article, and final approval of the version to be published.
Anne E. Holland: conception and design, critical review of important intellectual content, and final approval of the version to be published.
Cristiane S. de Oliveira: conception and design, data acquisition, and final approval of the version to be published.
Jessyca P.R. Selman: conception and design, data acquisition, and final approval of the version to be published.
Rejane A.S. de Castro: conception and design, data acquisition, data analysis and interpretation, and final approval of the version to be published.
Rodrigo A. Abensur: conception and design, data analysis and interpretation, critical review of important intellectual content, and final approval of the version to be published.
Samia Z. Rached: conception and design, data analysis and interpretation, critical review of important intellectual content, and final approval of the version to be published.
Alberto Cukier: conception and design, data analysis and interpretation, critical review of important intellectual content, and final approval of the version to be published.
Rafael Stelmach: conception and design, data analysis and interpretation, critical review of important intellectual content, and final approval of the version to be published.
Simone Dal Corso: conception and design, data acquisition and data analysis and interpretation, drafting the article, critical review of important intellectual content, and final approval of the version to be published.
FundingFundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP process no. 2016/13756-4).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the staff of the Postgraduate Program in Rehabilitation Sciences and the undergraduate research fellows. The authors would also like to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for funding this project.