Cardiovascular autonomic dysfunction is associated with the development of cardiovascular diseases, but little is known about it in children and adolescents with myelomeningocele.
ObjectiveThis study investigated the cardiovascular autonomic function in wheelchair-using children and adolescents with myelomeningocele.
MethodsTwenty-two participants were assigned to one of two groups: myelomeningocele group (n=11) and Control group (n=11). Heart rate variability and systolic blood pressure variability were collected in supine resting position using spectral analyses. Spontaneous baroreflex sensitivity was collected by time-domain through the sequence method.
ResultsAt rest, heart rate was higher in myelomeningocele group when compared to Control group (mean difference 22.1, 95% CI 4.82–39.40; p=0.01). The heart rate and systolic blood pressure variability parameters did not show differences between groups. However, myelomeningocele showed lower gain mean in baroreflex sensitivity (mean difference −4.5, 95% CI −8.47 to −0.60; p=0.02), when compared to Control.
ConclusionWheelchair-using children and adolescents with myelomeningocele presented differences in the autonomic cardiovascular function. This may be associated with hypomobility due to wheelchair dependence, and venous muscle pump insufficiency due to paraplegia.
Myelomeningocele (MMC) is a congenital spinal cord malformation, that occurred during development of the central nervous system.1 MMC is often associated with hindbrain herniation and hydrocephalus. MMC is also associated with peripheral neuropathy such as neuromuscular disability (weakness and paralysis), bone deformities and sensory loss.2 The average incidence is 1 to 10/1000 live births.3 Among these, approximately 76% reach adulthood (20–25 years). Life expectancy of individuals with chronic spinal cord injury is related to potentially treatable factors such as, cardiovascular, pulmonary and metabolic diseases.4 For patients with MMC, physical inactivity and hypomobility, decreased aerobic performance and excessive accumulation of body fat might lead to the development of co-morbidities, particularly cardiovascular diseases that are four times more prevalent in non-ambulatory patients, when compared to ambulatory patients.5
Prior to the development of cardiovascular anomalies, hemodynamic, morphological and functional changes are observed.6 The common cardiovascular autonomic control damage precedes the cardiovascular disease, which suggests that the evaluation of cardiovascular autonomic control should also be considered as an important predictor of heart morbidity and mortality in this population.7,8 The analyses of heart rate variability (HRV), blood pressure variability (BPV) and spontaneous baroreflex sensitivity (BRS) are valuable tools to investigate the cardiovascular autonomic function, since they are non-invasive and selective,9–11 and represent an important element in risk stratification of cardiovascular events.7,8,12
It is known that acute or chronic disturbances in body homeostasis, such as those observed in brain injury associated with MMC can damage the autonomic cardiovascular control, leading to further development of cardiovascular diseases.6 Furthermore, there are no studies in the literature investigating the cardiovascular autonomic nervous system in young patients with myelomeningocele. The main purpose of this study was to investigate the differences in parameters related to autonomic cardiovascular function with focus on BRS, heart rate and blood pressure (BP) variability in wheelchair-using children and adolescents with myelomeningocele.
MethodsParticipantsThis is a case–control study in which patients with MMC were matched by sex, body mass index (BMI), height, and age with a control group with asymptomatic participants. A convenience sample of children and adolescents with myelomeningocele was recruited from the Rehabilitation Center of the Ribeirão Preto Medical School Hospital. We invited 15 wheelchair-using MMC patients to participate. From those, 11 agreed to participate. Typical children and adolescents were recruited from private schools and non-governmental organizations from Ribeirão Preto, Brazil.
All experimental protocols were applied to both groups: the myelomeningocele group (MMC, n=11), composed of wheelchair-using children and adolescents, between the ages of 8–15 years and the Control group (Control, n=11), composed of healthy sedentary children and adolescents. The exclusion criteria, for both groups, were: (1) orthopedic or neurological upper limb disorders that would prevent the participant from performing the tests; (2) chronic (or acute) cardiovascular and/or respiratory disorders; (3) the use of drugs or any substance that would interfere with the cardiac autonomic function, and (4) level of physical activity classified as “active” or “very active” by the International Physical Activity Level Questionnaire (IPAQ). The study protocol was approved by the Human Research Ethics Committee of the Clinical Hospital of the Ribeirao Preto Medical School, Universidade de São Paulo (USP), Ribeirão Preto, SP, Brazil (protocol number: CAAE 14806513.0.0000.5440). All participants, and their parents, signed a consent form prior to the start of the study.
Clinical and anthropometric assessmentWe collected weight (in kilograms), height (meters) and body composition. The weight was measured using an electronic scale (WCS, Shanghai, China); the height was obtained by measuring the arm span with a measuring tape. The body composition was obtained by means of electrical bioimpedance equipment (Biodynamics 450®, São Paulo, SP, Brazil).
The R–R interval (RRi; measured by an electrocardiogram) and BP recordings for the analysis of HRV and BPV, were obtained at the Laboratory of Cardiovascular Physiology and Physiotherapy. All measurements were made by same examiner. The recordings were collected in a calm and quiet environment, with controlled temperature between 22°C and 23°C, with the participant lying in supine position.9 The participants and their parents/guardians were instructed to abstain from caffeine-containing beverages and physical activity for at least 24h prior to data collection, as well as have a good night of sleep on the night before the tests.13
For the electrocardiogram (ECG, ADInstruments, Sydney, Australia), the electrodes were positioned in the standard lead CM5. At the same time, a finger cuff was affixed around the participant's right middle finger to obtain the BP using a Finometer (Finapres Medical Systems, The Netherlands). The participants were encouraged to position themselves comfortably, and once settled, they were asked to remain still and not talk during the test. All children were kept in supine position for a total of 20min, 10min before recording of the data collection began and another 10min in order to record the stationary signals. The respiratory frequency was controlled by a chest strain gauge.
The analyses of HRV and BPV were performed using a computer software (CardioSeries v2.1 – http://sites.google.com/site/cardioseries) developed in the Physiology Department of the Ribeirão Preto Medical School of the Universidade de São Paulo (USP, Brazil). Stationary ten minute-series were used for the analysis of HRV and BPV. The stationarity of signals, which is prerequisite for spectral analysis, was visually monitored on a laptop screen. After signals stabilized, generally within a few minutes, BP and HR signals were recorded, with the participants in supine position for 10min.11 Ten-minute-long time series with RRi and systolic blood pressure (SBP) data values were resampled at 3Hz (at a constant interval of 333ms), using cubic spline interpolation for generating evenly-spaced series. The interpolated series with 50% overlap (Welch Protocol) were divided into continuous segments of 256 data points (85.3s) and were visually inspected for large artifacts or transients that could affect the results. Segments with noisy data were excluded from analysis.
The stationary character of the RRi and SBP values of each segment was visually inspected and those with artifacts, or transients, were excluded. Each stationary segment of RRi and SBP was subjected to spectral analysis by fast Fourier transform (FFT) with a Hanning window. The powers for RRi series were integrated in band of low (LF: 0.04–0.15Hz) and high (HF: 0.15–0.5Hz) frequencies. The results were expressed in absolute (ms2) and normalized units (un), while the SBP spectra was integrated in LF and expressed in absolute units (mmHg2).
Normalization of RRi oscillations was computed by dividing the absolute power of LF and HF component by the total power, minus the power of the very low frequency (VLF, <0.04Hz).14 Normalization was used to minimize the effects of total power variation for the values of LF and HF components.9,11 Additionally, the LF/HF ratio was also calculated in order to evaluate the cardiac sympathovagal balance.15
The BRS was assessed in the time-domain using the sequence method, as described by Di Rienzo et al.16 Custom computer software (CardioSeries v2.0, http://sites.google.com/site/cardioseries) scanned beat-by-beat time series of SBP and RRi, searching for sequences of at least 4 consecutive beats in which: increases in SBP were followed by RRi lengthening (up sequence) and decreases in SBP were followed by RRi shortening (down sequence), with a linear correlation higher than 0.8. The slope of the linear regression lines between SBP and RRi was used as a measure of spontaneous BRS.
Statistical analysisThe normal distribution was checked using Shapiro–Wilk and normal probability plot. Independent t-test was used to compare the groups. The values obtained from both groups were compared and presented in mean (SD) and mean difference [95% CI]. Statistical analyses were performed in SPSS, Inc. software version 23 (Chicago, IL, USA). For all analyses, a 5% significance level was used.
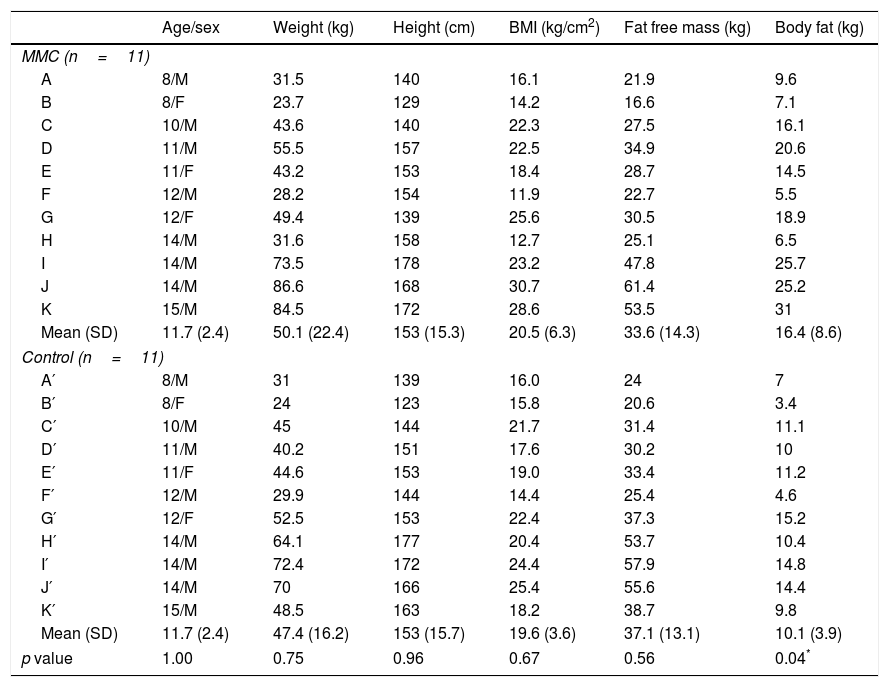
ResultsThe clinical characteristics of the participants in the two groups are shown in Table 1. The function levels of the lesion varied among the MMC participants as follows: sacral (n=5), low lumbar (n=2), high lumbar (n=1) and thoracic (n=3). There were no differences (p>0.05) in body weight, height, BMI and fat free mass between the groups. However, the MMC group showed higher fat mass when compared to the Control group (p=0.04) (Table 1).
Clinical characteristics of MMC and Control groups.
| Age/sex | Weight (kg) | Height (cm) | BMI (kg/cm2) | Fat free mass (kg) | Body fat (kg) | |
|---|---|---|---|---|---|---|
| MMC (n=11) | ||||||
| A | 8/M | 31.5 | 140 | 16.1 | 21.9 | 9.6 |
| B | 8/F | 23.7 | 129 | 14.2 | 16.6 | 7.1 |
| C | 10/M | 43.6 | 140 | 22.3 | 27.5 | 16.1 |
| D | 11/M | 55.5 | 157 | 22.5 | 34.9 | 20.6 |
| E | 11/F | 43.2 | 153 | 18.4 | 28.7 | 14.5 |
| F | 12/M | 28.2 | 154 | 11.9 | 22.7 | 5.5 |
| G | 12/F | 49.4 | 139 | 25.6 | 30.5 | 18.9 |
| H | 14/M | 31.6 | 158 | 12.7 | 25.1 | 6.5 |
| I | 14/M | 73.5 | 178 | 23.2 | 47.8 | 25.7 |
| J | 14/M | 86.6 | 168 | 30.7 | 61.4 | 25.2 |
| K | 15/M | 84.5 | 172 | 28.6 | 53.5 | 31 |
| Mean (SD) | 11.7 (2.4) | 50.1 (22.4) | 153 (15.3) | 20.5 (6.3) | 33.6 (14.3) | 16.4 (8.6) |
| Control (n=11) | ||||||
| A′ | 8/M | 31 | 139 | 16.0 | 24 | 7 |
| B′ | 8/F | 24 | 123 | 15.8 | 20.6 | 3.4 |
| C′ | 10/M | 45 | 144 | 21.7 | 31.4 | 11.1 |
| D′ | 11/M | 40.2 | 151 | 17.6 | 30.2 | 10 |
| E′ | 11/F | 44.6 | 153 | 19.0 | 33.4 | 11.2 |
| F′ | 12/M | 29.9 | 144 | 14.4 | 25.4 | 4.6 |
| G′ | 12/F | 52.5 | 153 | 22.4 | 37.3 | 15.2 |
| H′ | 14/M | 64.1 | 177 | 20.4 | 53.7 | 10.4 |
| I′ | 14/M | 72.4 | 172 | 24.4 | 57.9 | 14.8 |
| J′ | 14/M | 70 | 166 | 25.4 | 55.6 | 14.4 |
| K′ | 15/M | 48.5 | 163 | 18.2 | 38.7 | 9.8 |
| Mean (SD) | 11.7 (2.4) | 47.4 (16.2) | 153 (15.7) | 19.6 (3.6) | 37.1 (13.1) | 10.1 (3.9) |
| p value | 1.00 | 0.75 | 0.96 | 0.67 | 0.56 | 0.04* |
A–K, myelomeningocele participants (MMC); A′–K′, Control participants (Control); M, male; F, female; kg, kilogram; cm, centimetre.
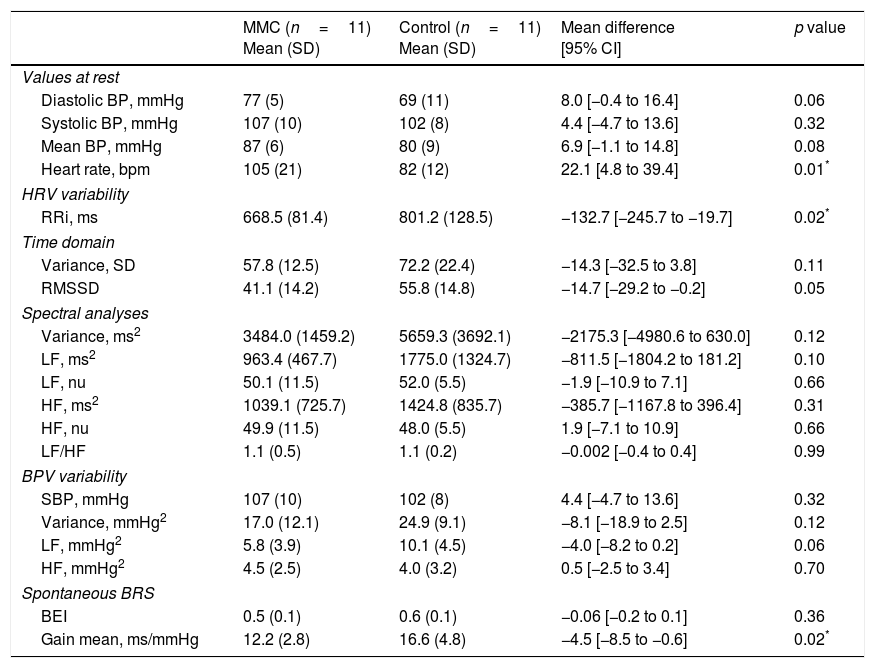
The MMC and Control groups showed similar BP values at rest. The HR values at rest were significantly higher for the MMC group when compared to the Control group (p=0.01). The groups did not show differences in HRV and BPV variability (p>0.05). MMC showed lower gain mean value obtained in the BRS (p=0.02), when compared to Control group (Table 2).
Blood pressure (BP) and heart rate (HR) at rest, heart rate variability (HRV), blood pressure variability (BPV) and spontaneous baroreflex sensitivity (BRS) for myelomeningocele (MMC) and Control groups.
| MMC (n=11) Mean (SD) | Control (n=11) Mean (SD) | Mean difference [95% CI] | p value | |
|---|---|---|---|---|
| Values at rest | ||||
| Diastolic BP, mmHg | 77 (5) | 69 (11) | 8.0 [−0.4 to 16.4] | 0.06 |
| Systolic BP, mmHg | 107 (10) | 102 (8) | 4.4 [−4.7 to 13.6] | 0.32 |
| Mean BP, mmHg | 87 (6) | 80 (9) | 6.9 [−1.1 to 14.8] | 0.08 |
| Heart rate, bpm | 105 (21) | 82 (12) | 22.1 [4.8 to 39.4] | 0.01* |
| HRV variability | ||||
| RRi, ms | 668.5 (81.4) | 801.2 (128.5) | −132.7 [−245.7 to −19.7] | 0.02* |
| Time domain | ||||
| Variance, SD | 57.8 (12.5) | 72.2 (22.4) | −14.3 [−32.5 to 3.8] | 0.11 |
| RMSSD | 41.1 (14.2) | 55.8 (14.8) | −14.7 [−29.2 to −0.2] | 0.05 |
| Spectral analyses | ||||
| Variance, ms2 | 3484.0 (1459.2) | 5659.3 (3692.1) | −2175.3 [−4980.6 to 630.0] | 0.12 |
| LF, ms2 | 963.4 (467.7) | 1775.0 (1324.7) | −811.5 [−1804.2 to 181.2] | 0.10 |
| LF, nu | 50.1 (11.5) | 52.0 (5.5) | −1.9 [−10.9 to 7.1] | 0.66 |
| HF, ms2 | 1039.1 (725.7) | 1424.8 (835.7) | −385.7 [−1167.8 to 396.4] | 0.31 |
| HF, nu | 49.9 (11.5) | 48.0 (5.5) | 1.9 [−7.1 to 10.9] | 0.66 |
| LF/HF | 1.1 (0.5) | 1.1 (0.2) | −0.002 [−0.4 to 0.4] | 0.99 |
| BPV variability | ||||
| SBP, mmHg | 107 (10) | 102 (8) | 4.4 [−4.7 to 13.6] | 0.32 |
| Variance, mmHg2 | 17.0 (12.1) | 24.9 (9.1) | −8.1 [−18.9 to 2.5] | 0.12 |
| LF, mmHg2 | 5.8 (3.9) | 10.1 (4.5) | −4.0 [−8.2 to 0.2] | 0.06 |
| HF, mmHg2 | 4.5 (2.5) | 4.0 (3.2) | 0.5 [−2.5 to 3.4] | 0.70 |
| Spontaneous BRS | ||||
| BEI | 0.5 (0.1) | 0.6 (0.1) | −0.06 [−0.2 to 0.1] | 0.36 |
| Gain mean, ms/mmHg | 12.2 (2.8) | 16.6 (4.8) | −4.5 [−8.5 to −0.6] | 0.02* |
Values are mean (SD) and mean difference [95% CI]; RRi, R–R interval; LF, low frequency; HF, high frequency; SBP, systolic blood pressure; nu, normalized units; ms, millisecond; mmHg, millimeter of mercury; BEI, baroreflex effectiveness index.
Wheelchair-using children and adolescents with myelomeningocele showed higher HR at rest values and lower BRS (gain mean), when compared to the Control group. The higher HR at rest observed in the MMC group may be associated with lower limb hypomobility and absence of intermittent activation of the muscle pump, even in the supine position. This may also induce peripheral vascular remodeling, due to low orthostatic stress, a phenomenon observed in paraplegics, as well as patients who are bed ridden for a long time.17 In this case, hypomobility and muscle pump inefficiency would cause a decrease in the venous return, which would affect the end-diastolic volume, reducing blood volume to increase HR at rest to preserve cardiac output. Consequently, the physiological compensatory mechanisms would become activated in an attempt to restore blood pressure and volume back to normal.18
The analysis of HRV, in the spectral frequency and time domain did not show any differences in cardiac autonomic modulation. In contrast, we found a significant difference in cardiac BRS in children and adolescents with myelomeningocele, when compared to their healthy counterparts. It is well known that BRS is not only a major determinant of the homeostatic response to alteration in blood pressure, but also an important predictor of cardiovascular risks, since it detects damage in cardiovascular reflex control which involves, mainly, the arterial baroreceptors.7 These receptors are sensitive to blood pressure variations, i.e. decreased blood pressure can reduce baroreflex activation and cause the heart rate to increase, restoring blood pressure levels. This process involves a complex network of interactions between the carotid and aortic receptors, afferent pathways, integration center in the brainstem, efferent pathways, and β-adrenergic and muscarinic receptors.19 While the complexity of these mechanisms is not fully understood, low BRS seems to indicate increase in cardiovascular risk. A prospective mortality study in patients with chronic diseases associated with the spinal cord, showed higher incidence of cardiovascular disease as the result of combined medical conditions (changes in the autonomic nervous system and motor paralysis) premature mortality and decrease in quality of life in this population.4 Low BRS can be used as an independent risk factor for sudden death, due to cardiovascular events linked to the dysfunction of the autonomic nervous system.7 However, most studies that address BRS and comorbid conditions use adult populations leaving an important gap in the literature for children and adolescents.
Moreover, little is known about this important predictor of cardiac morbidity and mortality in children and adolescents with myelomeningocele. One study conducted in Germany with typical (pre) adolescents showed BRS values similar to those seen in our control group.20 Among other possible causes, we hypothesized that the lower BRS observed in the MMC group would be a result of their restricted motor condition, with less possibilities of adjustments and postural changes and, consequently, adjustments in autonomic reflexes. Therefore, especially for those with less mobility, rehabilitation with mobilization and orthostatic posture is important to improve clinical symptoms. We also need to consider the severe decrease in cardiorespiratory fitness induced by this motor restriction.
Another important aspect to consider is that injury level may interfere in autonomic control. Individuals with high level lesions do not have tonic vasomotor outflow below the lesion level, and therefore would have reduced amplitude of SBP oscillations.19 On the other hand, those with lower level lesions have more systemic vasomotor tone, when compared to those with higher lesion levels, as they have less vascular tissue under sympathetic control that has been interrupted.21 To the extent of our knowledge, there are no studies in the literature analyzing autonomic function in children with myelomeningocele, thus some extrapolations were made to interpret the results obtained in this study. Autonomic nervous system involvement in individuals with high thoracic lesions (above T6) has been well documented in the literature, and it is the result of changes in the sympathetic pathways that are responsible for autonomic heart rate modulation. However, studies involving dysfunction in individuals with low lesions are still controversial. Castiglioni et al.,22 showed alterations in autonomic nervous system in individuals with low lesions (T8 and T10), explained by their sedentary lifestyle, since anatomically, the lesion did not directly interfere with the neural pathways. Serra-Añó et al.,23 showed alterations in autonomic nervous system with intact cardiac baroreflex arc and cardiac autonomic innervations in individuals with spinal cord injury and explained their findings in the same way. According to Phillips et al.,19 it is still unclear whether the vagal control of BRS is reduced in individuals with low lesion. From the hemodynamic point of view, cerebral perfusion is probably maintained and vagal control is preserved. This indicates that autonomic dysfunction is not only due to the interruption of spinal cord communication, but it is also affected by lifestyle, wheelchair dependence, compromised venous muscle pump and muscle paralysis.24 Although there is heterogeneity regarding the level of lesion in this study, they are all below T6, with no direct damage to the autonomic neural pathways. Furthermore, the fact that all individuals are wheelchair users and have the same level of physical activity (confirmed by IPAQ), the level of injury would not have been a confounding factor. There is need for further studies in order to identify the mechanisms behind this finding.
Although this is the first study providing autonomic function in children and adolescents with MMC, it has some limitations: (a) small sample of children and adolescents with different levels of lesion and physical activity; (b) no tilt-test, which could provide more information about the autonomic cardiovascular rearrangement due to orthostatic stress in children with MMC. However, we chose not to do it in this first study, since the environment where it was performed (outside the hospital) did not provide sufficient safety for eventual intercurrences that might occur to the detriment of the population studied. In summary, our results show that wheelchair-using children and adolescents with MMC show differences in cardiovascular autonomic function parameters when compared to their healthy counterparts. These findings indicate that their sedentary lifestyle, coupled with hypomobility (wheelchair dependence) and dysfunction of the venous muscle pump (caused by muscle paralysis) may be associated with the reduction of BRS. In a clinical practice, physical therapists would encourage the patients to move voluntarily several times during the day, and to take the orthostatic posture frequently. This would reduce the cardiovascular risk in MMC, increasing the demand on autonomic nervous system.
FundingThis work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2013/15425-7 and 2013/05936-4).
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank the volunteers who participated in this study, especially, patients with MMC and their caregivers, and the Laboratory of Cardiovascular Physiology and Physiotherapy for technical support.