The shoulder complex is highly loaded during swimming. No studies were found analyzing the changes in shoulder girdle strength in young swimmers, through the years.
ObjectiveTo analyze the changes in strength of internal rotators and external rotators of the arm, lower trapezius and supraspinatus and in the external rotators/internal rotators ratio in young elite swimmers through 3 years.
Methods31 adolescent elite swimmers (14–18 years, of both sexes) participated in the study. Isometric strength of the shoulder girdle muscles was performed using a handheld dynamometer during 3 years.
ResultsFor boys absolute data, internal rotators increased from the first to the second (p=0.0001; mean difference 45.6N; 95%CI 26.7–65.0) and third years (p=0.01; mean difference: 32.4; 95%CI: 9.3–55.5). Considering the weight-normalized data, internal rotators increased from the first to the second year (p<0.0001; mean difference: 0.52; 95%CI: 0.26–0.78), external rotators decreased from the first to the third year (p=0.003; mean difference: −0.33; 95%CI: −0.53 to −0.13) and from the second to the third year (p=0.0004; mean difference: −0.29; 95%CI: −0.46 to −0.12) and supraspinatus decreased from the second to the third year (p=0.006; mean difference: −0.17; 95%CI: −0.28 to −0.06). For girls, there were no significant differences in the absolute strength. Considering the weight-normalized data, lower trapezius decreased from the first to the third year (p=0.02; mean difference: −0.15; 95%CI: −0.27 to 0.03). Considering both sexes, the external rotators/internal rotators ratio decreased from the first to the second (p<0.0001; mean difference −0.12N; 95%CI −0.13 to −0.11) and third years (p<0.0001; mean difference −0.15N; 95%CI −0.16 to −0.14).
ConclusionMuscle imbalance can occur in the shoulder girdle in young swimmers in 3 years, with increased internal rotators and decreased external rotators and supraspinatus strength in boys, and decreased strength of the lower trapezius in girls. Attention should be given in young swimmers’ shoulder girdle muscle balance.
Swimming is one of the most popular sports worldwide and promotes several benefits for body and health. The shoulder complex is highly loaded during body propulsion in the water, with repetitive movements and no time to rest, which may explain the high incidence of shoulder pain and injuries in swimmers.1–5
During almost all swimming strokes, arm adduction and shoulder internal rotation are highly demanded. The muscles responsible for these movements become stronger than their antagonists, causing a possible muscle imbalance in these athletes.6,7 These changes in muscle balance may occur especially in internal and external rotators of the arm as these muscles help in the improvement of the swimming performance.8,9 Internal rotators (IR) and external rotators (ER) of the arm are responsible in providing stability and mobility to the glenohumeral, especially in overhead athletes.9–11 The literature frequently shows increased strength of the IR while the ER become weaker in competitive swimmers.2,8,10,11 Still considering the rotator cuff muscles, the supraspinatus is one of the main muscles responsible for stabilizing the humeral head in the glenoid fossa.12 This muscle has also an important role in positioning the arm during the hand entry and exit in the water during the swimming, in association with the middle deltoid, helping in the arm elevation and abduction.5,12 In this way, these muscles together are essential to protect the glenohumeral joint.
In addition to the glenohumeral muscles, the scapulothoracic muscles are also essential to improve the swimming performance and also to protect from injuries, since they should promote an adequate stabilization of the scapula in the thorax.4,12–14 The lower trapezius is considered an important muscle in swimmers. This muscle cooperates with the serratus anterior as a force couple for the scapular upward rotation and promotes scapular stabilization in association with the upper fibers of trapezius and rhomboid muscles.4,14–16 According to the literature, the LT is often shown to be weak in overhead athletes, which can predispose to scapular kinematics changes and this alteration can be found in the early stages of shoulder injuries.12,15
It is already known that years of practice and specialization in a specific sport can induce changes in the athlete's body. These changes are considered adaptations to their training and repetitive activity.17,18 In swimmers, studies were performed analyzing the muscles strength during a specific period such as competition, pre or post season.8,9,19 However, no studies were found analyzing the development and changes that may occur in the shoulder girdle strength in young swimmers through the years of adolescence. Furthermore, the literature shows that, considering the IR and ER, more important than their absolute strength, is the balance acquired by the both muscles, i.e. a balanced strength ratio.8 This knowledge could help in better understanding the changes and adaptations that occur in swimmers shoulder girdle strength through the years. Considering the critical role the humeral IR and ER, lower trapezius and supraspinatus play in the glenohumeral stability and mobility, it could be important to assess the strength of these muscles in swimmers, in order to recognize the athletes at risk for shoulder pain or injuries. Furthermore, it would be important to use an easy and non-expensive tool to assess these strength levels, since it could be used in the clinical practice.
Therefore, the purpose of this study was to analyze the changes that occur in the muscle strength of the IR and ER of the arm, supraspinatus and lower trapezius and external rotators/internal rotators ratio in young elite swimmers, boys and girls, in a period of 3 years. The hypothesis was that there would be an increase in the IR and supraspinatus strength levels through the 3 years, with a decrease in the ER and lower trapezius strength levels, for both sexes.
MethodsThis is a longitudinal cohort study. A convenience sample of forty-five young swimmers were invited to take part in the study. Fourteen participants were excluded due to shoulder pain (n=5) and other reasons (n=9). In this way, thirty-one adolescent elite swimmers (22 boys and 9 girls), between 14 and 18 years participated in the study. We excluded individuals who had neck or shoulder pain or dysfunction; surgical stabilization; previous fractures in the shoulder complex; positive impingement tests; systemic diseases involving the joints. At the beginning of the study, the participants used to swim 12–16h/week and to perform 4–6h of dryland training. With the progression of the study, they started swimming 16–20h/week and to perform 6–7h of dryland training. The swimmers participated in the competitions of the Flemish Swim Federation, including training camps abroad.
The research ethical committee of the Ghent University, Belgium, approved this study under the registration n°B670201421850. We provided a comprehensive information sheet for the individuals and who agreed signed an informed consent before enrollment and a parental or guardian signed consent was also obtained.
We performed the tests in the athletes’ first assessment of the year in 2013, 2014 and 2015. Same tester did all measurements in both sides. The right side was tested first and the same order was always followed: ER, IR, lower trapezius and supraspinatus. Three repetitions of 5s were performed for each muscle and side, with 5min of rest between repetitions. The measurements were recorded in Newton (N).
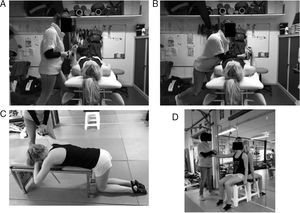
To test the ER and IR of the arm (Fig. 1A and B, respectively) the subject was positioned supine, with 90° of elbow flexion and the shoulder in neutral position. The HHD (Hand Held Dynamometer) was positioned in the distal part of the arm on the dorsal (ER) or ventral (IR) side of the forearm. The examiner stabilized the subjects arm at 0° of humeral rotation and asked them to perform internal or external rotation against the tester's resistance. Muscle strength was expressed in Newton, measured by the Hand Held Dynamometer. Based on their results, the ER/IR strength ratio was also calculated. To test the lower trapezius (Fig. 1C), an adaptation of Donatteli et al.20 strength test protocol was performed. The subject was kneeling on the floor, with knees and hips at 90° of flexion and the upper body supported by a bench (in order to avoid lower limb and trunk compensations during the test). The arm was placed diagonally overhead (145° of arm abduction) and in external rotation. The subjects should elevate their arm against the examiner resistance, who was applying resistance in the lateral and distal part of the forearm in downward direction. For the supraspinatus (Fig. 1D), the subject was seated in a chair with back support and the spine in a neutral position with shoulder elevation at 90° in the scapular abduction plane with humeral external rotation. The tester stabilized their arm and the subjects were asked to elevate the arm against the tester resistance. The HHD was positioned in the distal part of the humerus.
To add more information to the results a weight-normalized strength was also calculated, since adolescent athletes present a general increase in their muscle mass and performance.21
The average results of the 3 trials was calculated and analyzed using the SPSS version 17.0 (IBM Corp., Armonk, NY, USA). Kolmogorov–Smirnov test was used to test the normality of the data and the Bartlett test for the homogeneity of variance. Considering that all data were normally distributed, a 2-way ANOVA was conducted for each muscle and sex separately (boys and girls), comparing the strength through the 3 years. Dominance was considered as within factor and time (first year, second year and third year) as between factor. A post hoc analysis of Tukey HSD was used when necessary. A p value less than 0.05 was considered significant.
Studies already found an excellent reliability for measures obtained throughout the HHD, with ICCs above 0.8.22,23 In adolescents, strength measurements with HHD were also valid and reliable, with ICC ranging from 0.75 to 0.98.24
ResultsThe demographic data of the participants are presented in Table 1.
Characteristics of the participants per year.
| 1st year | 2nd year | 3rd year | ||||
|---|---|---|---|---|---|---|
| Boys (n=22) | Girls (n=9) | Boys (n=22) | Girls (n=9) | Boys (n=22) | Girls (n=9) | |
| Age (years) | 15.4±0.7 | 14.6±0.8 | 16.3±0.7 | 15.5±1.0 | 17.3±0.7 | 16.3±1.3 |
| Weight (kg) | 67.4±6.8 | 53.5±4.8 | 70.7±5.7 | 52.8±6.0 | 71.6±5.7 | 55.9±8.7 |
| Height (cm) | 180.4±4.5 | 166.7±4.0 | 182.4±4.6 | 167.3±3.2 | 185.9±5.5 | 168.0±5.6 |
Results are mean (±SD).
Considering the absolute strength (Table 2), no significant interaction of time×dominance was found for the ER of the arm (p=0.78), lower trapezius (p=0.98) and supraspinatus (p=0.98). Neither main effect of time was found for the ER (p=0.07), lower trapezius (p=0.08) and supraspinatus (p=0.44). For the IR of the arm, the interaction of time×dominance was not significant (p=0.99). However, there was a significant main effect of time (p=0.001), with an increase in the IR strength from the first to the second (p=0.0001; mean difference 45.6N; 95%CI 26.7 – 65.0) and to the third (p=0.01; mean difference 32.4N; 95%CI 9.3 – 55.5) year.
Boys’ shoulder girdle muscle strength.
| Muscle | Side | Mean (95%CI) | Mean difference (95%CI) | (p-Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st year | 2nd year | 3rd year | 1st – 2nd year | 1st – 3rd year | 2nd – 3rd year | Dme | T | I | ||
| ER Absolute (N) | D | 154.9 (140.2–169.5) | 158.3 (146.0–170.6) | 135.8 (123.7–147.8) | 3.5 (15.1–22.1) | −19.1 (−37.5 to 0.6) | −22.6 (−39.4 to 5.8) | 0.38 | 0.07 | 0.78 |
| ND | 144.4 (132.1–156.7) | 150.3 (138.5–162.1) | 136.7 (124.1–149.4) | 5.9 (10.6–22.4) | −7.6 (−24.7 to 9.4) | −13.5 (−30.3 to 3.2) | ||||
| D+ND | 149.6 (136.2–163.1) | 154.3 (142.3–166.3) | 136.3 (124.2–148.3) | 4.7 (−12.8 to 22.1) | −13.4 (−30.9 to 4.1) | −18.1 (−34.5 to −1.6) | ||||
| ER Normalized (N) | D | 2.52 (2.33–2.70) | 3.05 (2.87–3.22) | 2.8 (2.58–3.01) | 0.53 (0.28–0.78) | 0.28 (0.01–0.55) | −0.25 (−0.52 to 0.02) | 0.25 | 0.02 | 0.67 |
| ND | 2.49 (2.35–2.62) | 3.02 (2.86–3.17) | 2.77 (2.57–2.96) | 0.53 (0.33–0.73) | 0.28 (0.05–0.51) | −0.25 (−0.49 to −0.01) | ||||
| D+ND | 2.21 (2.05–2.36) | 2.17 (2.04–2.29) | 1.88 (1.75–2.00) | −0.04 (−0.24 to 0.16) | −0.33 (−0.53 to 0.13)* | −0.29 (−0.46 to 0.12)* | ||||
| IR Absolute (N) | D | 170.4 (155.4–185.5) | 216.1 (199.9–232.4) | 202.7 (181.8–223.5) | 45.7 (24.2–67.2) | 32.2 (7.2–57.2) | −13.5 (−39.2 to 12.2) | 0.79 | 0.0001 | 0.99 |
| ND | 168.1 (156.4–179.9) | 213.6 (199.4–227.8) | 200.7 (180.9–220.4) | 45.5 (27.6–63.4) | 32.5 (10.2–54.8) | −12.9 (−36.6 to 10.7) | ||||
| D+ND | 169.3 (156.1–182.5) | 214.9 (199.8–229.9) | 201.7 (181.9–221.5) | 45.6 (26.7–65.0)* | 32.4 (9.3–55.5)* | −13.2 (−37.3 to 10.9) | ||||
IR Normalized (N) | D | 2.29 (2.11–2.46) | 2.23 (2.09–2.36) | 1.88 (1.75–2.00) | −0.06 (−0.28 to 0.16) | −0.41 (−0.62 to 0.20) | −0.35 (−0.53 to −0.17) | 0.75 | <0.0001 | 0.99 |
| ND | 2.14 (1.98–2.28) | 2.12 (1.98–2.25) | 1.89 (1.75–2.02) | −0.02 (−0.21 to 0.17) | −0.25 (−0.44 to 0.06) | −0.23 (−0.41 to −0.05) | ||||
| D+ND | 2.5 (2.34–2.65) | 3.03 (2.86–3.19) | 2.78 (2.58–2.97) | 0.52 (0.26–0.78)* | 0.28 (0.04–0.52) | −0.25 (−0.50 to 0.00) | ||||
| SS Absolute (N) | D | 68.6 (62.4–74.8) | 73.2 (66.8–79.6) | 71.6 (65.4–77.8) | 4.6 (−4.0 to 13.3) | 3.0 (−5.6 to 11.7) | −1.6 (−10.3 to 7.0) | 0.36 | 0.44 | 0.98 |
| ND | 66.3 (61.5–71.0) | 70.7 (65.5–79.9) | 68.0 (62.6–73.3) | 4.4 (−2.4 to 11.3) | 1.7 (−5.3 to 8.7) | −2.8 (−10.0 to 4.5) | ||||
| D+ND | 67.4 (62.0–72.9) | 72.0 (66.2–77.7) | 69.8 (64.1–75.5) | 4.5 (−3.2 to 12.2) | 2.3 (−5.3 to 10.0) | −2.2 (−10.1 to 5.7) | ||||
| SS Normalized (N) | D | 1.01 (0.94–1.07) | 1.04 (0.96–1.11) | 0.99 (0.92–1.05) | 0.03 (−0.06 to 0.12) | −0.02 (−0.11 to 0.07) | −0.05 (−0.14 to 0.04) | 0.65 | 0.01 | 0.95 |
| ND | 0.98 (0.92–1.03) | 1.00 (0.94–1.05) | 0.94 (0.88–0.99) | 0.02 (−0.06 to 0.10) | −0.04 (−0.12 to 0.04) | −0.06 (−0.14 to 0.02) | ||||
| D+ND | 1.39 (1.30–1.47) | 1.43 (1.35–1.50) | 1.26 (1.17–1.34) | 0.04 (−0.07 to 0.15) | −0.13 (−0.25 to −0.01) | −0.17 (−0.28 to −0.06)* | ||||
| LT Absolute (N) | D | 95.4 (87.7–103.0) | 102.4 (95.0–108.7) | 92.0 (83.0–100.9) | 7.0 (−3.2 to 17.2) | −3.4 (−14.8 to 8.0) | −10.0 (−21.6 to 0.8) | 0.73 | 0.08 | 0.98 |
| ND | 92.8 (85.2–100.3) | 101.41 (94.1–108.7) | 91.2 (82.9–99.5) | 8.6 (−1.7 to 18.9) | −1.55 (−12.4 to 9.3) | −10.2 (−20.9 to 0.5) | ||||
| D+ND | 94.1 (86.6–101.5) | 101.88 (94.7–109.1) | 91.6 (83.2–100.0) | 7.8 (−2.2 to 17.9) | −2.5 (−13.4 to 8.4) | −10.3 (−21.0 to 0.4) | ||||
| LT Normalized (N) | D | 1.41 (1.32–1.49) | 1.44 (1.36–1.51) | 1.27 (1.17–1.36) | 0.03 (−0.09 to 0.15) | −0.14 (−0.26 to 0.02) | −0.17 (−0.29 to 0.05) | 0.26 | 0.45 | 0.97 |
| ND | 1.37 (1.28–1.45) | 1.43 (1.34–1.51) | 1.26 (1.17–1.34) | 0.06 (−0.06 to 0.18) | −0.11 (−0.23 to 0.01) | −0.17 (−0.23 to −0.05) | ||||
| D+ND | 0.99 (0.92–1.05) | 1.01 (0.94–1.07) | 0.96 (0.90–1.01) | 0.02 (−0.07 to 0.11) | −0.03 (−0.11 to 0.05) | −0.05 (−0.13 to 0.03) | ||||
Results are mean (95%CI) and mean difference (95%CI). CI, confidence interval; Dme, main effect of dominance; T, main effect of time; I, interaction dominance×time; D, dominant; ND, non-dominant; ER, external rotators; IR, internal rotators; SS, supraspinatus; LT, lower trapezius; N, Newton.
Regarding the weight-normalized data (Table 2), for the ER of the arm, no significant interaction of time×dominance was found (p=0.67). However, there was a significant main effect of time (p=0.002), with a decrease in the ER strength from the first to the third year (p=0.003; mean difference −0.33N; 95%CI −0.53 to 0.13) and from the second to the third year (p=0.004; mean difference −0.29N; 95%CI −0.46 to 0.12). For the IR of the arm, the interaction of time×dominance was not significant (p=0.99) However, there was a significant main effect of time (p<0.0001), with an increase in the IR strength from the first to the second (p<0.0001; mean difference 0.52N; 95%CI 0.26–0.78) year. For the lower trapezius, no significant interaction of time×dominance was found (p=0.97) neither main effect of time (p=0.45). For the supraspinatus, no significant interaction of time×dominance was found (p=0.95). However, there was a significant main effect of time (p=0.009), with a decrease in the supraspinatus strength from the second to the third year (p=0.006; mean difference −0.17N; 95%CI −0.28 to −0.06).
Girls shoulder girdle strengthConsidering the absolute strength (Table 3), no significant interaction of time×dominance was found for any of the muscles, such as ER (p=0.97), IR (p=0.80), lower trapezius (p=0.95) and supraspinatus (p=0.87). Neither main effect of time was found for any of the muscles, such as ER (p=0.70), IR (p=0.95), lower trapezius (p=0.97) and supraspinatus (p=0.48).
Girls’ shoulder girdle muscle strength.
| Muscle | Side | Mean (95%CI) | Mean difference (95%CI) | (p-Value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st year | 2nd year | 3rd year | 1st – 2nd year | 1st – 3rd year | 2nd – 3rd year | Dme | T | I | ||
| ER Absolute (N) | D | 113.5 (106.0–121.0) | 118.7 (111.0–126.4) | 111.8 (105.1–118.4) | 5.2 (−12.0 to 22.3) | −1.8 (−17.8 to 14.2) | −7.0 (−23.2 to 9.3) | 0.57 | 0.70 | 0.97 |
| ND | 111.4 (100.8–121.9) | 113.1 (104.3–122.0) | 108.3 (99.0–117.5) | 1.8 (−20.2 to 23.7) | −3.2 (−25.5 to 19.2) | −4.9 (−25.3 to 15.4) | ||||
| D+ND | 112.4 (97.7–127.2) | 116.0 (102.0–129.9) | 110.0 (97.0–123.5) | 3.5 (−15.2 to 22.2) | −2.5 (−20.8 to 15.9) | −5.9 (−23.8 to 11.9) | ||||
| ER Normalized (N) | D | 2.94 (2.80–3.07) | 2.98 (2.63–3.25) | 2.73 (2.46–2.99) | 0.04 (−0.3 to 0.38) | −0.21 (−0.49 to 0.07) | −0.25 (−0.65 to 0.15) | 0.73 | 0.61 | 0.95 |
| ND | 2.83 (2.58–3.07) | 2.97 (2.71–3.22) | 2.83 (2.57–3.08) | 0.14 (−0.18 to 0.46) | 0.00 (−0.32 to 0.32) | −0.14 (−0.47 to 0.19) | ||||
| D+ND | 2.10 (1.88–2.31) | 2.20 (1.97–2.42) | 1.98 (1.75–2.20) | 0.10 (−0.18 to 0.38) | −0.12 (−0.40 to 0.16) | −0.22 (−0.51 to 0.07) | ||||
| IR Absolute (N) | D | 157.3 (141.9–172.6) | 158.1 (131.0–185.1) | 152.3 (129.1–175.5) | 0.8 (−27.8 to 29.4) | −5.0 (−30.6 to 20.6) | −5.8 (−38.6 to 27.1) | 0.97 | 0.95 | 0.88 |
| ND | 150.7 (135.4–166.0) | 157.7 (135.2–180.3) | 158.3 (134.0–182.6) | 7.0 (−18.0 to 32.0) | 7.6 (−18.8 to 34.0) | 0.6 (−30.0 to 31.0) | ||||
| D+ND | 154.0 (139.5–168.4) | 157.9 (133.9–181.8) | 155.3 (132.3–178.2) | 3.9 (−21.8 to 29.6) | 1.3 (−23.6 to 26.2) | −2.6 (−33.1 to 27.9) | ||||
IR Normalized (N) | D | 2.13 (1.93–2.32) | 2.26 (2.02–2.49) | 2.02 (1.83–2.20) | 0.13 (−0.15 to 0.41) | −0.11 (−0.36 to 0.14) | −0.24 (−0.51 to 0.03) | 0.96 | 0.34 | 0.78 |
| ND | 2.08 (1.81–2.34) | 2.14 (1.90–2.37) | 1.95 (1.68–2.21) | 0.06 (−0.26 to 0.38) | −0.13 (−0.47 to 0.21) | −0.19 (−0.52 to 0.14) | ||||
| D+ND | 2.88 (2.68–3.07) | 2.97 (2.68–3.25) | 2.77 (2.51–3.02) | 0.09 (−0.23 to 0.41) | −0.11 (−0.40 to 0.18) | −0.20 (−0.53 to 0.13) | ||||
| SS Absolute (N) | D | 57.6 (54.5–60.6) | 52.8 (44.8–60.7) | 51.5 (40.9–62.0) | −4.8 (−12.6 to 3.0) | −6.1 (−16.2 to 4.0) | −1.3 (−13.5 to 10.9) | 0.40 | 0.48 | 0.87 |
| ND | 52.8 (50.6–55.0) | 52.2 (44.8–59.7) | 47.7 (38.4–57.1) | −0.4 (−7.6 to 6.7) | −5.0 (−13.9 to 3.8) | −4.6 (−15.6 to 6.6) | ||||
| D+ND | 55.2 (52.0–58.3) | 52.5 (45.1–59.9) | 49.6 (39.9–59.3) | −2.7 (−10.1 to 4.7) | −5.6 (−15.0 to 3.8) | −5.0 (−15.6 to 6.5) | ||||
| SS Normalized (N) | D | 1.08 (1.01–1.14) | 0.99 (0.91–1.06) | 0.92 (0.78–1.05) | −0.09 (−0.19 to 0.01) | −0.16 (−0.30 to −0.02) | −0.07 (−0.21 to 0.07) | 0.81 | 0.91 | 0.92 |
| ND | 0.99 (0.95–1.02) | 0.98 (0.91–1.04) | 0.85 (0.73–0.96) | −0.01 (−0.07 to 0.05) | −0.14 (−0.25 to −0.03) | −0.13 (−0.25 to −0.01) | ||||
| D+ND | 1.75 (1.25–2.24) | 1.69 (1.27–2.10) | 1.64 (1.25–2.02) | −0.06 (−0.66 to 0.54) | −011 (−0.69 to 0.47) | −0.05 (−0.57 to 0.47) | ||||
| LT Absolute (N) | D | 98.7 (60.7–136.6) | 91.7 (61.9–121.5) | 91.2 (64.3–118.1) | −7.0 (−51.3 to 37.4) | −7.4 (−50.2 to 35.3) | −0.5 (−37.4 to 36.5) | 0.85 | 0.97 | 0.95 |
| ND | 90.5 (63.5–117.5) | 89.7 (60.0–119.5) | 93.9 (64.6–123.1) | −0.8 (−37.7 to 36.2) | 3.4 (−33.2 to 40.0) | 4.1 (−34.2 to 42.5) | ||||
| D+ND | 94.6 (63.9–125.2) | 90.7 (62.1–119.3) | 92.5 (65.5–119.6) | −3.9 (−42.4 to 34.7) | −2.0 (−39.6 to 35.5) | 1.8 (−34.4 to 38.0) | ||||
| LT Normalized (N) | D | 1.83 (1.19–2.46) | 1.71 (1.27–2.14) | 1.62 (1.24–1.99) | −0.12 (−0.83 to 0.59) | −0.21 (−0.89 to 0.47) | −0.09 (−0.62 to 0.44) | 0.19 | 0.01 | 0.72 |
| ND | 1.68 (1.24–2.11) | 1.67 (1.23–2.10) | 1.67 (1.24–2.08) | −0.01 (−0.57 to 0.55) | −0.01 (−0.56 to 0.54) | 0.00 (−0.55 to 0.55) | ||||
| D+ND | 1.03 (0.96–1.09) | 0.98 (0.91–1.04) | 0.88 (0.76–0.99) | −0.05 (−0.14 to 0.04) | −0.15 (−0.27 to 0.03)* | −0.10 (−0.22 to 0.02) | ||||
Results are mean (95%CI) and mean difference (95%CI). CI, confidence interval; Dme, main effect of dominance; T, main effect of time; I, interaction dominance×time; D, dominant; ND, non-dominant; ER, external rotators; IR, internal rotators; SS, supraspinatus; LT, lower trapezius; N, Newton.
Regarding the weight-normalized data (Table 3), no significant interaction of time×dominance was found for the ER (p=0.95), IR (p=0.78), for the lower trapezius (p=0.72) and for the supraspinatus (p=0.92). Neither for the main effect of time for the ER (p=0.61), IR (p=0.34), and the supraspinatus (p=0.91), with exception of the lower trapezius which had a significant main effect of time (p=0.01), with a decrease in the lower trapezius strength level from the first to the third year (p=0.0241; mean difference −0.15N; 95%CI −0.27 to 0.03).
ER/IR ratioFor the ER/IR ratio, both sexes were kept together. The main effect of time was significant (p<0.0001), suggesting a decrease in the ratio from the first to the second (p<0.0001; mean difference −0.12N; 95%CI −0.27 to 0.03) and to the third years (p<0.0001; mean difference −0.15N; 95%CI −0.16 to −0.14) (Table 4).
External rotation/internal rotation strength ratio (ER/IR).
| Mean (95%CI) | Mean difference (95%CI) | (p-Value) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dominance | 1st year | 2nd year | 3rd year | 1st – 2nd year | 1st – 3rd year | 2nd – 3rd year | Dme | T | I | |
| ER/IR | D | 0.86 (0.81–0.90) | 0.74 (0.70–0.77) | 0.70 (0.66–0.73) | −0.12 (−0.18 to −0.06) | −0.16 (−0.22 to −0.10) | −0.04 (−0.09 to 0.01) | 0.14 | <0.0001 | 0.90 |
| ND | 0.82 (0.77–0.86) | 0.71 (0.68–0.73) | 0.69 (0.65–0.72) | −0.11 (−0.16 to −0.06) | −0.13 (−0.18 to −0.08) | −0.02 (−0.07 to 0.03) | ||||
| D+ND | 0.84 (0.83–0.84) | 0.72 (0.71–0.72) | 0.69 (0.68–0.69) | −0.12 (−0.13 to −0.11)* | −0.15 (−0.16 to −0.14)* | −0.03 (−0.07 to 0.01) | ||||
Results are mean (95%CI) and mean difference (95%CI). CI, confidence interval; Dme, main effect of dominance; T, main effect of time; I, interaction dominance×time; D, dominant; ND, non-dominant; ER, external rotators; IR, internal rotators; SS, supraspinatus; LT, lower trapezius; N, Newton.
Although several studies have analyzed shoulder girdle strength in swimmers, to our knowledge this was the first to analyze the shoulder girdle strength in a longer period in both sexes, separately. The present study aimed to analyze changes and progression of humeral ER, humeral IR, lower trapezius and supraspinatus muscle strength and the ER/IR strength ratio in young elite swimmers in a period of 3 years, for boys and girls. The results are partially in agreement with our hypothesis, since there was an increase in the IR and a decrease in the ER and supraspinatus strength, however only for the boys. While for the girls, there was only a decrease in the lower trapezius strength.
In the present study, an increase in the IR strength level through the years, for both absolute and weight-normalized values, was found for the boys. This is in agreement with Batalha et al.9 who found an increase in IR strength in young swimmers through a season. Ramsi et al.8 also found an increase in IR strength with no similar gain of the ER strength through 12 weeks in young swimmers, suggesting that this could be a risk factor for shoulder injuries in the future. The present study, diverging in part from the literature, have found that through 3 years, no significant changes occurred in the ER strength in the absolute values. When considering the weight-normalized values, the ER had a decrease in the strength from the first and second to the third year. The literature shows that associated to an increase in the IR strength, there is also an increase in the ER.8,9 However, the IR increases in a disproportionate way compared with their antagonists, becoming stronger than the ER.8,9,25
According to the literature, more important than the absolute IR and ER strength, is their ratio balance, ER/IR.8,9,26 This ratio is considered of extreme importance, especially for overhead athletes. To have a balanced ER/IR ratio, the normative values already observed in non-athletes and tennis players should be between 66 and 75%, whereas lower than this could be considered as a severe imbalance, with risk to develop shoulder injuries.21,27,28 In swimmers this ratio is still contradictory, however some studies have already found that this ratio ranged between 73 and 77% in young swimmers, and it decreased from the baseline to the end of the season, showing that the shoulder rotators imbalance occurs in young swimmers.8,9 In the present study, a decrease in this ratio was established through the first to the second and third year, suggesting an imbalance in shoulder rotators through the years without taking the sex into account. However, even suggesting an imbalance, it cannot be considered severe, since the lowest ratio was around 69%. Nevertheless, special attention should be paid, since this imbalance has already been related to glenohumeral instability and shoulder impingement.28
Another muscle that plays an important role for glenohumeral stability during overhead sports is the supraspinatus. This is one of the main rotator cuff muscles responsible for maintaining the humeral head in the glenoid fossa.5 In the present study, boys presented decreased strength of the supraspinatus from the second to the third year when considering the weight-normalized values. This muscle, due to its essential role in the shoulder function and especially due to its location, is highly affected by pathologies, such as tendinopathies and tears.29–32 The literature also shows that tendons present a lack of circulation and poor nutrition. In the case of repetitive microtraumas, since it does not present a properly heal, it can develop chronic injuries, which may progress to tendon rupture in the future.30,32
Regarding the lower trapezius, it is considered an important scapular stabilizer.33,34 During overhead sports, a well-stabilized scapula is essential in order to perform the upper limb motions in the most efficient and safe way, with minimal risk of injury. Since swimming is a sport that requires a great demand of the upper limb, a great scapular stabilization is necessary to perform the stroke. However, when considering the boys, no differences were found in the lower trapezius strength through the years, meanwhile the girls presented a decrease in lower trapezius strength from the first to the third year for the weight-normalized data. Kibler (1998)15 suggests that the lower trapezius has an important role in the force couple, promoting the scapular upward rotation, helping to avoid the impingement during the swimming stroke.14
Furthermore, an interesting finding of the present study was the absence of muscle strength changes (IR, ER and supraspinatus) for the girls through the years. The literature shows that boys normally present higher increase in strength during adolescence than girls do, especially for the upper limb.35–37 This may be attributed to the fact that boys at this age present increased testosterone levels,37 while girls normally have already achieved a specific osseous and muscle maturation. The developmental process normally happens for girls earlier than for boys, whereas at this age they usually already had their menarche. In this way, muscle strength changes may be more difficult to be found in girls at this age.
A finding that may represent a risk for these young swimmers, especially for the boys, is that, there was a significant increase in the IR strength. Furthermore, the ER instead of increasing or keeping at the same level, it decreased through the years. Considering the absence of muscle strength changes found in the girls, an interesting subject is that in the adult life, women present a higher prevalence of shoulder pain when compared to men.38–40 The influence of these findings in these young athletes lives must be seen with such caution, since it is expected that in athletes, especially high level athletes, an increase should happen in the muscle strength through the years.8,9
In this way, the present study found that shoulder muscle imbalance can occur in young swimmers, in a period of 3 years of practice and competition. The literature has already shown two facts that can maybe explain this imbalance, (1) the greater recruitment of IR during the swimming,41 and (2) swimming promotes a stress in the glenohumeral capsular ligaments, which can cause an instability and can change the muscle capacity to produce strength.42 For this reason, it would be important to promote a balance in the glenohumeral and scapulothoracic muscles in young swimmers, focusing in particular on strengthening of ER, supraspinatus and lower trapezius, in order to prevent shoulder injuries and pain in these athletes.14,27,43
One of the greatest strengths of this study was the possibility to follow these young swimmers through 3 years, since it is already shown in the literature that competitive activities can cause some musculoskeletal adaptations, especially in children and adolescents.44 The extent of these adaptations may be considered as an injury risk factor for these young athletes, due to their intensive athletic activity, where adaptations in strength and flexibility may not always be beneficial.44,45 Furthermore, this study used the hand held dynamometer to measure the muscles strength, which is an easy and non expensive tool and can be used in the clinical practice. The study present some limitations, such as (1) the lack of strength measurement of the other scapulothoracic muscles (serratus anterior and upper trapezius), however the strength measurement of these muscles is not recommended to be performed using the manual resistance as we used in the present study, since these muscles are too strong for the tester to resist manually; (2) lack of injury registration, making impossible a deeper analysis, relating possible alterations to pain or injury in these athletes; (3) no information on developmental status of these adolescents, such as menarche, hormonal levels and puberty stages. In this way, future researches should provide information about the other scapulothoracic muscles and risk of injuries related to the shoulder muscle imbalances found in young swimmers.
In conclusion, our results suggests that muscle imbalance can be found in shoulder girdle muscles in young swimmers in a period of 3 years of practice. An increased IR strength and decreased ER and supraspinatus strength in boys and decreased lower trapezius strength in girls were found. In this way, special attention should be paid in young swimmers shoulder muscle balance, in order to understand the influence of the swimming practice in these muscles strength. Furthermore, sex should be taken into account when performing preventive or rehabilitation protocols for young swimmers.
Conflicts of interestThe authors declare no conflicts of interest.
The first author acknowledges the scholarship provided by São Paulo Research Foundation (FAPESP/BEPE – 2014/26412-6).