Early breast cancer diagnoses have enabled better therapeutic responses and better patient prognoses. However, sarcopenia, may develop as a result of this oncological disease, through the release of inflammatory cytokines.
ObjectivesDetermine the prevalence of sarcopenia and associations with weekly energy expenditure and physical activity (PA) levels in female patients with breast cancer at the time of diagnosis in a reference oncology hospital.
MethodsCross-sectional study of patients with breast cancer stages IA to IIIC between June 2021 and June 2022. Sarcopenia was classified as “probable” when patients had low muscle strength, “confirmed” when both low strength and low muscle mass were detected, and “severe” when these two factors were associated with low physical performance. Muscle strength was assessed using dynamometry, muscle mass through calf circumference and physical performance using the Timed Up and Go test. PA level was determined by the International Physical Activity Questionnaire. Binary logistic regressions were performed between sarcopenia and PA levels, while linear regressions were performed for weekly energy expenditure.
ResultsNinety-two women were evaluated. Sarcopenia prevalence was determined as probable in 5.4 % and confirmed in 14.1 % of participants. It was observed that women classified as having probable or confirmed sarcopenia were older than those who were not sarcopenic. No significant differences concerning weekly energy expenditure and PA levels between groups were observed.
ConclusionLess than a quarter of the assessed patients were diagnosed with probable or confirmed sarcopenia, and none were diagnosed with severe sarcopenia. No associations were observed between sarcopenia, weekly energy expenditure or PA levels.
Breast cancer is the most common tumor detected in women worldwide.1 According to Global Cancer Statistics, a worldwide breast cancer incidence of over 2.26 million was noted in 2020, leading to about 684,996 deaths.2 In Brazil, the National Cancer Institute (INCA) has estimated 73,610 new breast cancer cases for each year of the three-year 2023–2025 period.3 The early diagnosis of this disease, however, enables better therapeutic responses and higher survival rates.4
Several complications of breast cancer are noted, which can include pain and stiffness in the joints in patients undergoing treatment. Hormonal therapies can harm bone health, increasing the risk of fractures. Fatigue, also occurs in up to 94 % of patients after the diagnosis of breast cancer.5 And breast cancer treatments, often leads to low levels of physical activity (PA) following treatment.6 “Physical activity is defined as any bodily movement produced by skeletal muscles that results in energy expenditure”.7 Lack of physical activity can cause deconditioning and reduced physical functionality.8 In this sense, a sedentary lifestyle can, in turn, lead to long-term effects, such as unfavorable body compositions, such as sarcopenia.9
This condition can also occur as a direct oncological consequence, as evidence suggests that tumor masses are responsible for the production of inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin 1 (IL-1), both of which are associated to sarcopenia development.10 In oncology, the presence of sarcopenia has been associated with reduced efficacy of antineoplastic therapies, postoperative complications, and worse overall survival for several tumors.11
The term sarcopenia was first employed in 1989 by Inwin Rosenberg, who described this condition as a muscle mass decline associated to the aging process.12 In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) proposed a new sarcopenia definition, consisting in a muscular and skeletal disorder characterized by progressive reductions in both muscle mass and strength associated with an increased likelihood of adverse effects, including falls, fractures, physical morbidities, impaired ability to perform daily living activities and mortality.13 In this sense, a prospective analysis of 461 patients recently diagnosed with early-stage or metastatic cancer and who underwent curative surgery and had not undergone chemotherapy or radiotherapy before, observed a prevalence of sarcopenia of 17 %,14 while a meta-analysis relating to female patients, evaluated at any time after the diagnosis of breast cancer, indicated that those classified as sarcopenic had a 68 % higher risk of mortality compared to non-sarcopenic patients.15
The EWGSOP2 Guideline considers three factors for a sarcopenia diagnosis, namely low muscle strength, low muscle quality, and low physical performance. Probable sarcopenia is categorized as a low muscle strength condition, while sarcopenia is established by the association of low muscle strength and low muscle mass. Furthermore, this condition is categorized as severe when both low muscle strength and low muscle mass are associated to low physical performance.16
Given the complications and severity related to the development of sarcopenia in patients with cancer11,13 and given the few studies on the prevalence of this clinical condition, which analyze women who have at least already undergone surgical treatment for breast cancer,14,17,18 it is relevant to conduct this survey prior to cancer treatments, in this context, this study's primary objective is to evaluate the prevalence of sarcopenia and as a secondary objective to observe whether there are associations with weekly energy expenditure and PA levels in women evaluated by a physical therapy service at the time of breast cancer diagnosis in a reference cancer hospital.
MethodsThis study comprises a cross-sectional assessment belonging to the project entitled “Prehabilitation program for women indicated for surgical breast cancer treatment”, approved by the INCA Ethics and Research Committee (approval number 4.576.731), conducted at the HCIII/INCA Physical therapy sector between June 2021 and June 2022. Women over 18 years old presenting with breast cancer, enrolled for treatment at the HCIII/INCA, and who attended their first physical therapy consultation after being admitted to the institution, were included in the study. Women with a previous cancer diagnosis, presenting tumor stages 0 or IV, who performed physical exercise for at least 90 min a week, who were unable to answer the study questionnaires or who exhibited orthopedic, neurological, cardiorespiratory, and/or renal dysfunctions making it impossible to perform the functional tests were excluded. Patients who met the eligibility criteria were invited to participate, and after agreeing, signed a Free and Informed Consent Form (FICF). Sarcopenia was assessed through muscle strength, muscle mass, and physical performance measurements, and PA levels were assessed through a specific questionnaire. Sociodemographic characteristics (age, ethnicity, marital status, and education) and clinical characteristics (clinical staging and body mass index (BMI)) were obtained from electronic medical records.
Sarcopenia was classified as probable when low muscle strength was present, as confirmed when the patient exhibited both low muscle strength and low muscle mass and severe when low muscle strength and low muscle mass were associated with low physical performance.16 Patients who did not meet any of these criteria were classified as nonsarcopenic.
Muscle strength was assessed by palm gripping using a 5030 J1 dynamometer (Jamar®, United States) according to the American Society of Hand Therapy recommendations and considering the highest value obtained on the dominant side.19 Low muscle strength (for women) was set as < 16 kg/f.16
Muscle mass was measured by calf circumference (CC), an alternative method cited in the EWGSOP2 consensus when gold standard tests are unavailable,16 and Trussardi Fayh and de Sousa,20 indicate that CC measurements are accurate as a muscle mass marker when evaluating sarcopenia in older patients with cancer.20 The largest calf perimeter was used, using an inextensible tape TR-4010 (Sanny®, Brazil), adjusted along the entire calf length without compressing the skin. Measurements were recorded to the nearest centimeter,21 and a correction calculation was performed according to the BMI of each patient.22 Low muscle mass was set as ≤ 33 cm.23
Physical performance was evaluated by the Timed Up and Go Test (TUGT),24 where participants seated in a 46 cm high chair 25 were asked to get up, walk a 3 m circuit and return to their seat.16 Low physical performance was detected when the time to perform the test was ≥ 20 s 16
Physical activity levels were assessed using the short version of the International Physical Activity Questionnaire (IPAQ), which assesses PA frequency and time spent over a week. The energy expenditure calculated for each activity was estimated as metabolic equivalents (METs)/minute per week, with PA levels classified as high, moderate, or low. High PA levels are achieved when vigorous-intensity activity is performed on at least 3 days a week, achieving a total PA score of at least 1500 METs/minutes per week, or when the patient conducts a 7-day performance of a combination of walking, moderate or vigorous intensity, reaching a minimum of 3000 METs/minutes per week. Moderate PA levels comprise one of the following: 3 or more days of vigorous-intensity activity and/or walking for at least 30 min/day, 5 or more days of moderate-intensity activity and/or walking at least 30 min/day or 5 or more days of any combination of walking, moderate-intensity, or vigorous-intensity activities, achieving a minimum of 600 METs/minutes per week. Low PA levels is defined as not meeting any of these criteria.26
The data analysis included both probable and confirmed sarcopenia cases. Descriptive analyses were performed for sociodemographic and clinical patient characteristics. Central tendency and dispersion measures were applied to continuous variables, while analysis of variance tests (ANOVA) or the Mann-Whitney U independent sample test were performed for determining differences between groups according to the data distribution. Categorical variables were described by relative and absolute frequency distributions and compared between groups using the chi-square test. Binary logistic regressions (odds ratio (OR)) were used to evaluate the associations of sarcopenia with PA levels, and linear regressions were performed to evaluate the associations of sarcopenia with weekly energy expenditure. In both cases, multiple regressions were performed for the adjusted analysis, with age being applied as an adjustment variable. Differences were considered statistically significant when p < 0.05.
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 20.0 software.
ResultsA total of 102 patients were first assessed, with 10 excluded, seven due to stage 0 and three due to stage IV cancer. The final patient count was therefore 92 females with breast cancer, with a mean age of 54.6 (± 11.5) years and the majority had clinical stages of IIB and IIIA (39.1 %).
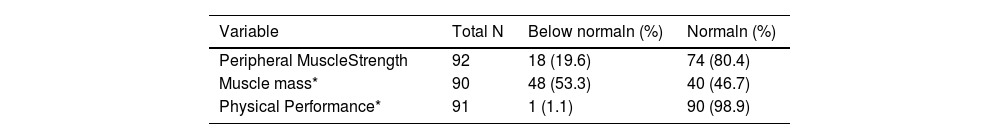
According to the peripheral muscle strength (PMS) assessments, 19.6 % exhibited low PMS. Low muscle mass was noted in 53.3 % of the patients. Only one patient presented low physical performance (Table 1). Probable sarcopenia was therefore determined in 5.4 % (n = 5) of all patients, confirmed sarcopenia, in 14.1 % (n = 13), and no sarcopenia, in 80.4 % (n = 74). No patient was classified as severely sarcopenic (Table 2).
Muscle strength, muscle mass, and physical performance of females with breast cancer at the time of breast cancer diagnosis.
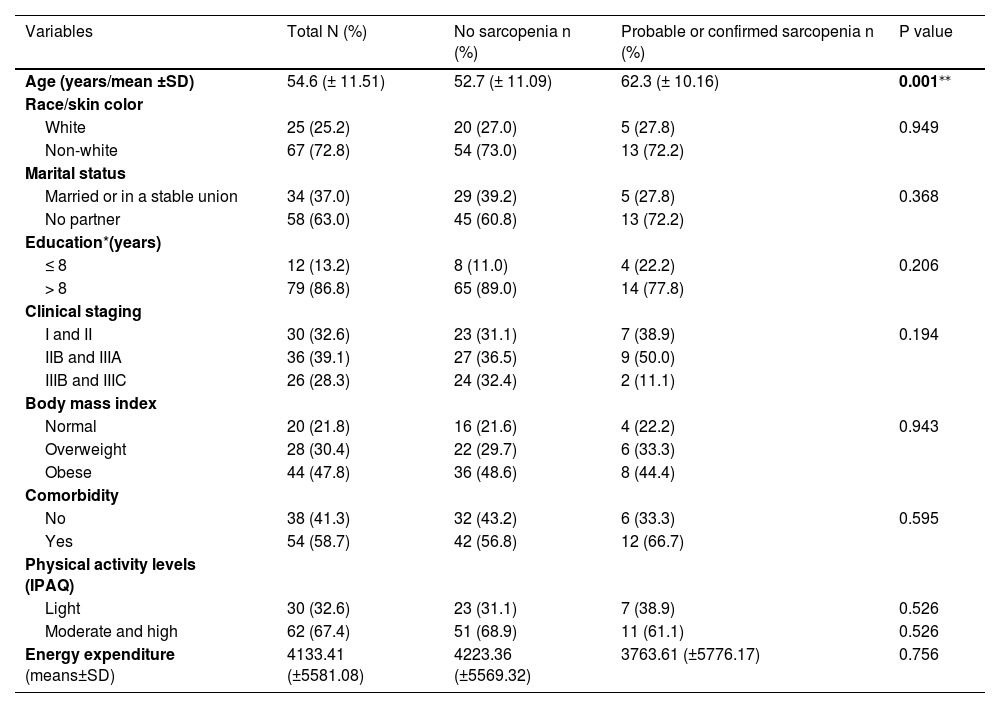
Patient sociodemographic and clinical data according to sarcopenia classification are depicted in Table 3. The mean age of non-sarcopenic patients was 52.7 (± 11.09) years, increasing to 62.3 (± 10.16) years in patients presenting probable and confirmed sarcopenia (p = 0.001). No differences regarding demographic and clinical characteristics according to sarcopenia profile were observed. Most patients were non-white (72.8 %), living without a partner (63.0 %), and with over 8 years of education (86.8 %). Concerning clinical characteristics, most patients presented locally advanced breast cancers ≥ IIB (67.4 %) and were overweight or obese (78.2 %). With regard to PA levels, 67.4 % of the assessed patients presented moderate and high PA levels. As for energy expenditure, patients in the non-sarcopenic group displayed a weekly energy expenditure of 4223.36 (±5569.32) METs/minutes per week, while patients with probable and confirmed sarcopenia reported lower weekly energy expenditures, with a metabolic equivalent value of 3763.61 (±5776.17) METs/minutes per week, albeit with no statistical difference from the non-sarcopenic group (Table 3).
Sociodemographic and clinical data between the non-sarcopenic (n =74) and sarcopenic (probable or confirmed (n = 18)) groups in females with breast cancer at the time of breast cancer diagnosis.
| Variables | Total N (%) | No sarcopenia n (%) | Probable or confirmed sarcopenia n (%) | P value |
|---|---|---|---|---|
| Age (years/mean ±SD) | 54.6 (± 11.51) | 52.7 (± 11.09) | 62.3 (± 10.16) | 0.001⁎⁎ |
| Race/skin color | ||||
| White | 25 (25.2) | 20 (27.0) | 5 (27.8) | 0.949 |
| Non-white | 67 (72.8) | 54 (73.0) | 13 (72.2) | |
| Marital status | ||||
| Married or in a stable union | 34 (37.0) | 29 (39.2) | 5 (27.8) | 0.368 |
| No partner | 58 (63.0) | 45 (60.8) | 13 (72.2) | |
| Education*(years) | ||||
| ≤ 8 | 12 (13.2) | 8 (11.0) | 4 (22.2) | 0.206 |
| > 8 | 79 (86.8) | 65 (89.0) | 14 (77.8) | |
| Clinical staging | ||||
| I and II | 30 (32.6) | 23 (31.1) | 7 (38.9) | 0.194 |
| IIB and IIIA | 36 (39.1) | 27 (36.5) | 9 (50.0) | |
| IIIB and IIIC | 26 (28.3) | 24 (32.4) | 2 (11.1) | |
| Body mass index | ||||
| Normal | 20 (21.8) | 16 (21.6) | 4 (22.2) | 0.943 |
| Overweight | 28 (30.4) | 22 (29.7) | 6 (33.3) | |
| Obese | 44 (47.8) | 36 (48.6) | 8 (44.4) | |
| Comorbidity | ||||
| No | 38 (41.3) | 32 (43.2) | 6 (33.3) | 0.595 |
| Yes | 54 (58.7) | 42 (56.8) | 12 (66.7) | |
| Physical activity levels (IPAQ) | ||||
| Light | 30 (32.6) | 23 (31.1) | 7 (38.9) | 0.526 |
| Moderate and high | 62 (67.4) | 51 (68.9) | 11 (61.1) | 0.526 |
| Energy expenditure (means±SD) | 4133.41 (±5581.08) | 4223.36 (±5569.32) | 3763.61 (±5776.17) | 0.756 |
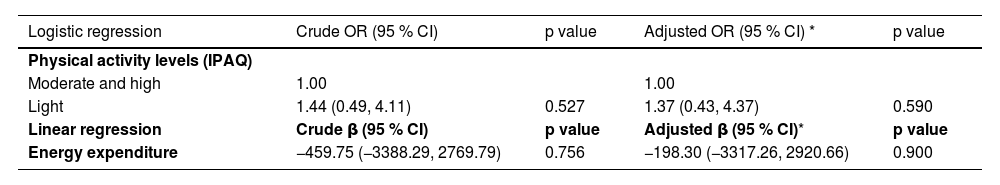
No associations between sarcopenia (probable or confirmed) and PA levels or weekly energy expenditure in the crude or age-adjusted analyses for females with breast cancer at the time of diagnosis were observed (Table 4).
Associations between sarcopenia (probable or confirmed), physical activity levels, and age-adjusted weekly energy expenditure in females with breast cancer at the time of breast cancer diagnosis (n = 92).
| Logistic regression | Crude OR (95 % CI) | p value | Adjusted OR (95 % CI) * | p value |
|---|---|---|---|---|
| Physical activity levels (IPAQ) | ||||
| Moderate and high | 1.00 | 1.00 | ||
| Light | 1.44 (0.49, 4.11) | 0.527 | 1.37 (0.43, 4.37) | 0.590 |
| Linear regression | Crude β (95 % CI) | p value | Adjusted β (95 % CI)* | p value |
| Energy expenditure | −459.75 (−3388.29, 2769.79) | 0.756 | −198.30 (−3317.26, 2920.66) | 0.900 |
Herein, sarcopenia was determined through muscle strength and muscle mass. Physical performance was used to classify the severity of sarcopenia, as proposed in EWGSOP2 2019.16 A total of 5.4 % of the evaluated females with breast cancer in this study presented probable sarcopenia and 14.1 %, confirmed sarcopenia, with a significant mean age of over 60 when compared to nonsarcopenic patients. No associations were observed between PA levels, weekly energy expenditure, and sarcopenia.
One systematic review analyzed patients presenting different types of tumors at different clinical stages, especially digestive ones, evaluating sarcopenia prevalence before the beginning of cancer treatment. Women with breast cancer, however, were not included. A total of 6894 patients who underwent sarcopenia assessments through computed tomography (CT) and bioimpedance were assessed in 35 studies, with a sarcopenia prevalence of 38 %,11 well above that found in our study (14.1 %), which can be explained by the small "n" sample. Similar frequencies to the findings reported here in this study, were indicated in two other studies with a breast cancer population. The first employed the EWGSOP2 cutoff criteria to bioimpedance and dynamometry assessment, reporting sarcopenia in 17 % of 461 patients with cancer and in 11.5 % of females with breast cancer after surgical treatments.14 The second and more recent study employed the same bioimpedance and dynamometry criteria, reporting sarcopenia in 13.9 % (n = 122) of all patients with breast cancer, who had undergone at least surgical or neoadjuvant treatment, while none of the individuals in the control group, composed of cancer free women, were sarcopenic.17
In the present study, a muscle strength median of 20 kg/f (± 8) was obtained, with 19.6 % (n = 18) of the assessed patients presenting low muscle strength. One study assessing PMS in 439 cancer patients reported a mean of 20.2 kg/f (± 6.6) in sarcopenic patients, increasing to 28.0 kg/f (± 9.5) (p < 0.001) in non-sarcopenic patients.27 A significant difference in medians was also observed in the present study, increasing from 13 kg/f (±2) in patients with probable and confirmed sarcopenia to 22 kg/f (± 8) in non-sarcopenic patients (p < 0.001). Another study evaluated 108 older patients with cancer with a predominance of colorectal cancer (27.8 %) and gastric cancer (22.2 %), with 13.9 % (n = 15) of females with breast cancer, and 74.1 % (n = 80) previously having undergone oncological treatment. A slightly higher low muscle strength frequency was observed by hand dynamometry, of 39.8 %, when compared to the present study (19.6 %).20
Regarding muscle mass, 53.3 % (n = 48) of the patients investigated herein presented low muscle mass measurements. The previously cited study on 108 older patients with cancer reported that 25.9 % (n = 28) of the investigated patients with cancer presented poor muscle quality when assessed by calf circumference measurements, while a CT assessment indicated a 24.1 % prevalence. The authors, thus, indicate that calf circumference measurements are accurate as a muscle mass marker when evaluating sarcopenia in older patients with cancer, in addition to being a quick, cheap, and easy measurement, which may aid in sarcopenia diagnosis. 20
Physical performance assessed by the TUGT in the present study indicated a total median of 8.7 s (±2.94), with only one patient presenting low physical performance. Another study employing the TUGT as an evaluation parameter for 72 patients with breast cancer aged ≥ 70 reported an average TUGT time of 11.5 s. Seventeen of the patients averaged 12 s and were considered at functional risk for sarcopenia, due to an applied cutoff point of ≥ 12 s.28 The present study, however, employed a cutoff point ≥ 20 s, as recommended by the EWGSOP2.16 Another study evaluated 34 patients with a mean age of 72.62 (± 5.66) years, who had already undergone breast cancer treatment and reported falls, noting that patients with reports of falls exhibited a TUGT performance time of 11.3 s.29
In addition to the aforementioned outcomes, this study also investigated patient PA levels. Non-sarcopenic patients presented a weekly energy expenditure of 4223.36 (±5569.32) METs, while patients with probable and confirmed sarcopenia expended 3763.61 (±5776.17) METs/minutes per week. No studies assessing PA levels in patients prior to breast cancer treatment are, however, available for comparisons. Concerning assessments during treatment, a weekly energy expenditure of 2315.5 (± 3513.2) METs/minutes per week was reported in a multicenter study on 339 patients undergoing surgical breast cancer treatment with a mean age of 50.4, with 64.3 % (n = 218) having already undergone chemotherapy and 68.4 % (n = 232), adjuvant radiotherapy. As in the present study, patients' PA levels were also evaluated categorically, where 33.0 % of all patients were considered inactive, 17.4 % considered active, and 49.6 %, minimally active,30 the latter similar to the 32.6 % frequency determined herein for low PA levels, although in patients not yet submitted to oncological treatment.
The mean patient age in the present study was 54.6 (± 11.51) years. The probable and confirmed sarcopenia group, however, presented the highest mean age, of 62.3 (± 10.16) years, while non-sarcopenic patients presented a mean age of 52.7 (± 11.09) years (p = 0.001). In a meta-analysis of 6 studies with 5497 women diagnosed with breast cancer, in three studies the average age was 54.1, 55.0, and 56.0 years, similar to the average found in this study, and a higher risk of mortality was found for sarcopenic patients aged 55 or over (pooled HR = 1.99, 95 % CI: 1.05, 3.78, P = 0.017).18 In a previously mentioned cross-sectional study, the average age of women with breast cancer was 49.3 years, lower than that found in this study, and it was seen that sarcopenic patients with breast cancer were older (55.8 ± 12.5) than non-sarcopenic patients (46.6 ± 9.1) (p < 0.05), a result also shown here.¹⁷
The BMI assessment carried out herein revealed that 47.8 % (n = 44) of the patients were obese, with a BMI ≥ 30 kg/m², with two (4.5 %) classified as likely sarcopenic and six (6.5 %) confirmed as sarcopenic, categorizing them as sarcopenic obese patients (data not tabulated). According to the EWGSOP2, sarcopenic obesity occurs in cases of excess adipose tissue and declining muscle tissue, with risks noted as increasing with age.16 A retrospective study on 338 women with a mean age of 51 with early-stage breast cancer and undergoing chemotherapy demonstrated a sarcopenia prevalence in CT-diagnosed patients of 17 % (n = 58), with a high frequency of obese women (40 %; n = 135) with BMI ≥ 30 kg/m²,31 similar to the present study.
The strong points of this study consist in the evaluation of sarcopenia in women with breast cancer at the time of diagnosis, as there are few studies with this objective. The study contributes in a practical and clinical way by employing accessible resources, such as dynamometry, calf circumference measurements, and TUGT, without the need for more robust and costly methods for public health services, showing the early detection of sarcopenia, as this condition is associated with worse cancer treatment outcomes, worsening post operative complications and chemotherapy-induced toxicities.32 This condition also results in reduced quality of life concerning physical and mental health and greater chances of disabilities, including in instrumental daily living activities, also interfering with overall cancer patient survival.20 Therefore, future studies should focus on early sarcopenia diagnosis and patients at risk for sarcopenia at the time of breast cancer diagnosis and evaluate potential interventions through PA levels for those who need it the most.
Limitations for this study include the fact that this assessment comprised a cross-sectional study that does not allow for the establishment of any temporality between PA levels and sarcopenia, as the patients were evaluated at the same time with a small sample size may not be enough to statistically detect significant differences. Furthermore, the obtained data can only be generalized to populations presenting similar socioeconomic, clinical characteristics, and level of physical activity. Another limitation of this study is the assessment of muscle mass carried out by calf circumference, as it is a subjective analysis, carried out by more than one evaluator, even though we made the correction by BMI, even so, Trussardi Fayh and de Sousa20, indicated that calf circumference measurements are accurate as a muscle mass marker when evaluating sarcopenia in older patients with cancer,20 and the EWGSOP2 consensus citing it as an alternative when there is no other diagnostic method available, however the recommendation to confirm sarcopenia is through performing tests such as DXA, BIA, MRI, or CT scan.16
ConclusionLess than a quarter of the population assessed herein was categorized as presenting probable or confirmed sarcopenia and no patient was classified as severely sarcopenic. No associations between sarcopenia, PA levels or energy expenditure in females with breast cancer at the time of diagnosis were detected.
All authors contributed to the study conception and design. Data collections were performed by NTFS and MVSC. Analysis were performed by DMT and SSA. All authors commented on previous versions and approved the final manuscript.