There is considerable overlap between pain referral patterns from the lumbar disc, lumbar facets, the sacroiliac joint (SIJ), and the hip. Additionally, sciatic like symptoms may originate from the lumbar spine or secondary to extra-spinal sources such as deep gluteal syndrome (GPS). Given that there are several overlapping potential anatomic sources of symptoms that may be synchronous in patients who have low back pain (LBP), it may not be realistic that a linear deductive approach can be used to establish a diagnosis and direct treatment in this group of patients.
ObjectiveThe objective of this theoretical clinical reasoning model is to provide a framework to help clinicians integrate linear and non-linear clinical reasoning approaches to minimize clinical reasoning errors related to logically fallacious thinking and cognitive biases.
MethodsThis masterclass proposes a hypothesis-driven and probabilistic approach that uses clinical reasoning for managing LBP that seeks to eliminate the challenges related to using any single diagnostic paradigm.
ConclusionsThis model integrates the why (mechanism of primary symptoms), where (location of the primary driver of symptoms), and how (impact of mechanical input and how it may or may not modulate the patient's primary complaint). The integration of these components individually, in serial, or simultaneously may help to develop clinical reasoning through reflection on and in action. A better understanding of what these concepts are and how they are related through the proposed model may help to improve the clinical conversation, academic application of clinical reasoning, and clinical outcomes.
Identifying the specific anatomic source of symptoms in patients who have low back pain (LBP) may not be an attainable goal. The convergence of several different neurologic structures in anatomic regions surrounding the lumbar spine in this patient population makes identifying the source of referred symptoms problematic. There is considerable overlap between pain referral patterns from local lumbar pathoanatomic sources related to the disc and facets, and pathoanatomic sources outside of the lumbar spine associated with the sacroiliac joint (SIJ), and the hip.1–6 Based on studies utilizing the patient's history, physical exam findings, diagnostic imaging, epidural injections, and facet blocks, among patients seeking care for LBP with or without leg pain, approximately 82% have spine pathology. Still, only 61–65% will present with isolated spine findings.7,8 An additional 12.5–17.5% of patients are likely to present with some combination of spine and hip or spine and SIJ dysfunction, and 8–9% may present with hip or SIJ pathology without spine pathology.7,8 The combination of all three areas presenting as pain generators appears to be less than 2%.7 Overall, it seems that 10–15% of cases of LBP may present with an undefined anatomic source of pain.7,8 The overall prevalence of non-lumbar pathology is approximately 14.5% for SIJ pathology, and 12.5% for hip pathology.8
Sciatic like symptoms may originate from the lumbar spine,9 SIJ,10 or secondary to extra-spinal sources such as piriformis syndrome11 or deep gluteal syndrome (GPS).12 Piriformis syndrome is reported to account for the majority of cases of sciatic symptoms outside the lumbar spine from a musculoskeletal origin.13 GPS has been proposed as a diagnosis to include anatomic structures that may be involved with nerve compression outside of the lumbar spine. These anatomic structures include the piriformis muscle, fibrous bands in gluteal muscles, the hamstring muscles, the gemelli-obturator internus complex, vascular abnormalities, and space-occupying lesions.12 It is estimated that 6–17% who present with sciatic symptoms have GPS.14
Controversy exists regarding the anatomic source of a patient's symptoms. This is secondary to the use of different reference tests and gold standards for establishing a diagnosis in patients that experience LBP.15 The cause of the patient's symptoms may not be attributable to kinesiopathological or pathoanatomic variables,16–18 and there is a high prevalence of pathoanatomic findings in asymptomatic individuals.19 Additional challenges include misleading test metrics and overly complicated diagnostic labels.20 While substantial effort has been placed on the creation of diagnostic labels to direct treatment,21,22 it is also clear that the current treatment-based classifications are not able to classify patients 25–34% of the time23,24 and current movement-based classifications fare no better than general guidelines for patients with chronic LBP.25
Clinicians that treat patients with LBP are also interested in seeing if the patient's primary complaint at any given time is modifiable through mechanical input.26 Given that there are several overlapping potential anatomic and non-anatomic sources of symptoms that may be synchronous in patients who suffer from LBP, it may not be realistic that a linear deductive approach can be used to classify, diagnose, and direct treatment in this group of patients. One of the significant challenges for any clinical reasoning approach in a clinical or academic setting is how to account for the lack of certainty when combining linear (deductive) and non-linear (inductive) reasoning processes when performing clinical reasoning. Exam findings in patients who have LBP are not dichotomous. They shift the probability of something being true. We would therefore like to propose a hypothesis-driven, probabilistic, mechanism-based approach to managing LBP that eliminates the challenges related to using any single diagnostic paradigm.
The purpose of this theoretical clinical reasoning model is to provide a framework to help clinicians integrate linear and non-linear clinical reasoning approaches to minimize clinical reasoning errors related to logically fallacious thinking and cognitive biases. This model is based on the integration of the presented evidence with the opinion and clinical experience of the authors. It seeks to serve as a vehicle through which the clinician may integrate and apply the best available evidence in a clinical context. “Whenever you find yourself on the side of the majority, it is time to pause and reflect.” — Mark Twain
The generation of hypotheses is an iterative process that begins when the clinician first meets the patient and evolves during the examination process. The evolution occurs as data are progressively gathered, organized, and prioritized using Bayesian reasoning. Bayesian reasoning involves the application of probability theory to abductive and inductive reasoning.27 As data are progressively collected, it shifts the probability that something is true. There is a growing body of literature to support that theoretical instruction in Bayesian concepts improves the estimation of post-test probabilities during the reasoning process.28–30 Bayesian reasoning includes the reasoning related to musculoskeletal pain irritability.31 Musculoskeletal pain irritability is pain that continues after the symptom provoking activities that produced a patient's symptoms have stopped.32 This concept may be an essential consideration as the clinician plans how to perform the physical exam following the medical history. Given that most testing is provocative, the clinician must do the least amount of testing to attain the greatest amount of information. If the patient has high symptom irritability, symptom alleviation may be the most powerful tool to use at that time and defer a more comprehensive physical exam to a later date, while still offering meaningful information regarding symptom behavior. This hypothesis testing strategy may include using a comparable sign related to the patient's primary symptoms as a benchmark for the hypothesis testing strategy to determine if the hypothesis is probabilistically accurate.31 This involves symptom modification that tests a hypothesis by determining if an examination procedure or intervention changes (increases, decreases, or stays the same) the patient's primary symptoms.26 Rationally, the thought that any procedure or intervention “cause” the modification in symptoms can never be assumed to be completely accurate without the risk of creating cognitive biases and logical fallacies. The principles of any treatment system for LBP may be used to generate and modify hypotheses at any given time during the examination process. The hypotheses, however, must be tested and verified probabilistically within the context of the patient.
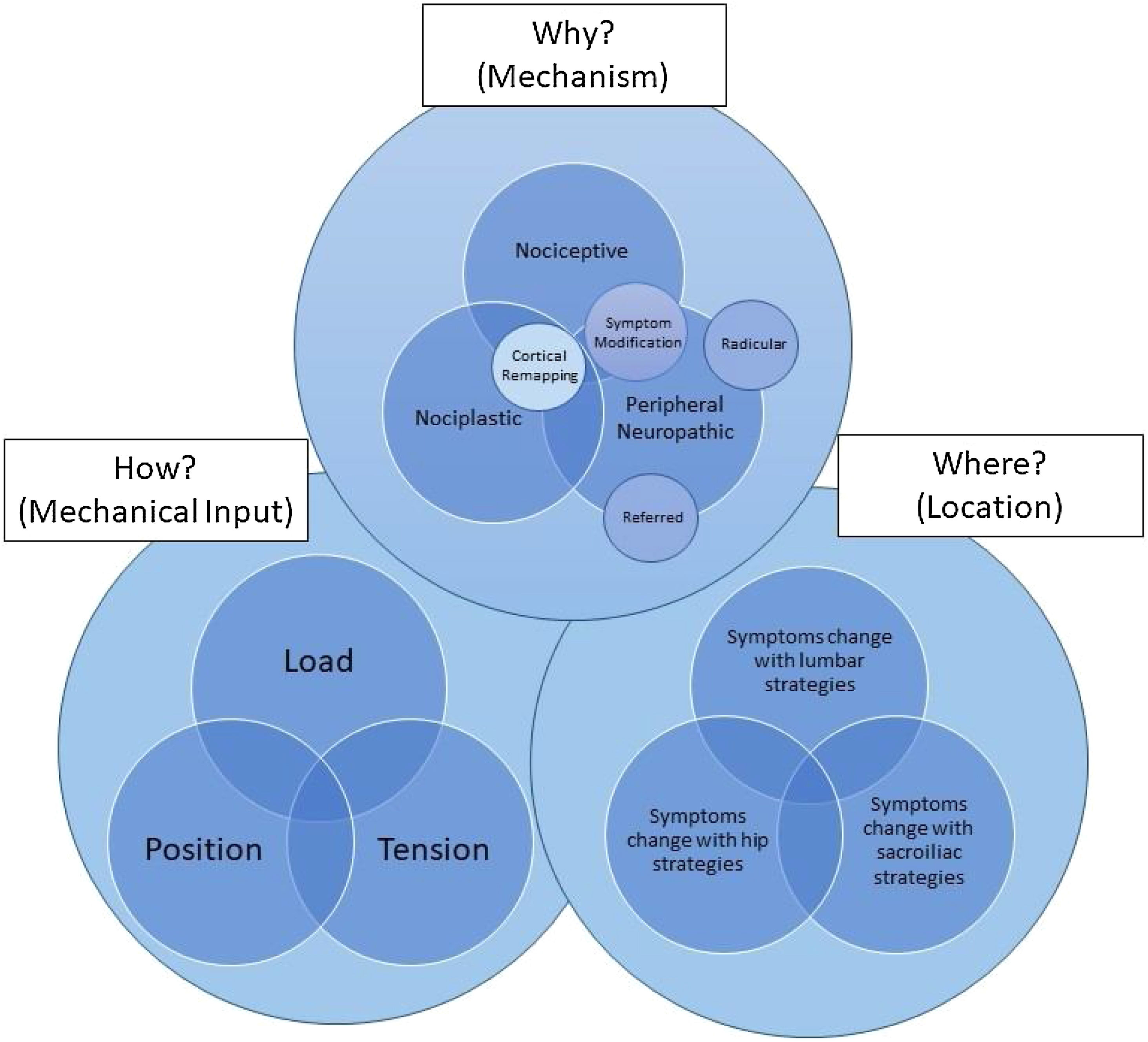
Using mechanism-based classifications: identifying the whySmart et al.33 established the discriminative validity of three classification systems for individuals that suffer from LBP that were derived through a Delphi consensus of clinical indicators. They then used statistical modeling based on patient symptoms to identify predominant sources of a patient’s symptoms who have LBP. Through this approach, they identified a mechanisms-based classification for musculoskeletal pain that included: 1) central sensitization;34 2) peripheral neuropathic (radicular or referred), 35 and 3) nociceptive.36 The ability to identify the predominant mechanism-based classification is reliable in patients with nonspecific cervical pain (kappa=.84 (95% CI: .65, 1.00), p < .001).37 Chimenti et al.38 have modified the work of Smart et al.33; by changing the name of the central sensitization classification to nociplastic, placed each mechanism in an overlapping Venn diagram to illustrate that all three sources of symptoms may occur at the same time, and have put the interaction of these classifications in the context of the movement system and psycho-social factors.38 They have also linked these mechanisms to physical therapy interventions that may be most appropriate to address the patient's symptoms.38 The validity of this classification has not been established in a clinical setting.39 This classification system may, however, be a valuable tool for generating a hypothesis related to the patient's dominant mechanism-based symptoms at any given time. Attempting to modify a patient's symptoms, testing the hypothesis with an exam strategy or intervention that should change their symptoms if the hypothesis is correct, may provide a robust and reasoned approach when applied iteratively within and between treatment sessions.
Nociplastic (central sensitization)In the absence of red flag findings, nociplastic symptoms are characterized by pain that is disproportionate, non-mechanical, unpredictable, and diffuse.34 Patients with nociplastic symptoms often have maladaptive behaviors related to the presence of negative beliefs (fear-avoidance), lack of positive beliefs related to self-efficacy, and dyskinetic movement related to kinesiophobia.34 This cluster of findings was found to have a sensitivity of 91.8% and a specificity of 97.7%.34 If a patient has nociplastic mediated pain, it is expected that the physical exam may not significantly change the patient's primary symptomatic complaints. These findings strongly suggest that the patient should be educated that their pain experience may not be driven through mechanical input, especially early during the rehabilitation process. The focus should be on using techniques to increase the patient's function without increasing their symptoms. Numerous techniques have been proposed to be useful in this biopsychosocial context. A discussion of the assessment and management of LBP using the biopsychosocial model is beyond the intent and scope of this clinical reasoning model. Although there is evidence in support of the importance of the model in determining etiological and prognostic factor variables for managing patients with LBP, the optimal method of assessment and treatment utilizing this approach has yet to be identified.40 The technique that matches the patient's and clinician's beliefs is likely the best choice of intervention at any given time. If the patient does not have any red flag findings or the cluster of findings consistent with nociplastic symptoms, this mechanism can be ruled out. If the patient responds to interventions with an increase in symptoms related to mechanical input, nociplastic symptoms may be a secondary contributing factor, and nociceptive and peripheral neuropathic contributions to the patient's primary complaints should be ruled out.
Peripheral neuropathic (referred and/or radicular)Peripheral neuropathic pain has been described as pain that occurs secondary to mechanical deformation or dysfunction in a peripheral nerve.41,42 Patients with primary peripheral neuropathic pain have symptoms that are referred in a dermatomal or cutaneous distribution and have a history of nerve injury, pathology, or mechanical compromise of the nerve with symptom provocation with mechanical testing. This cluster of findings was found to have a sensitivity of 86.3% and a specificity of 96.0%.35 For patients with potential referred symptoms, hypotheses should be formulated related to the primary mechanism (why) and structure (where) that is responsible for the symptoms. If the primary mechanism and structure are accurate, the clinician should be able to predict how the referred symptoms should change (increase or decrease) with alterations in position, load, and tension through the structure. The pattern of symptom provocation and alleviation can be used to educate the patient on what to avoid and to identify what may be used to modulate the patient's primary symptoms.
For patients with radicular symptoms, it becomes crucial to identify the potential primary structure(s) that may be responsible. If the patient has a lateral lumbar shift, a shift correction could be used to determine if the patient's primary complaint centralizes or peripheralizes.43,44 In the absence of a lumbar shift, the patient's reports of their symptomatic response (increase and/or decrease) to posture, position (flexion or extension), repetitive motion (flexion or extension), mid-range motion, load, and tension may provide valuable clues to generate hypotheses related to the most likely source of the radicular symptoms at any given time. These hypotheses should be tested to determine if these variables peripheralize or centralize the patient's radicular symptoms.45 An inability to centralize patient's symptoms suggests a poor prognosis to non-surgical management and may indicate the need for a referral in the case of non-responsive progressive symptoms.46
NociceptiveNociceptive pain is localized to an area of injury or dysfunction.36 Provocation and/or alleviation are identifiable and proportionate, match known mechanical and anatomical distributions, symptoms are usually intermittent and start with the onset of movement or mechanical provocation.36 The quality of symptoms may be a constant dull ache or a throb at rest.36 This group of patients should not have pain associated with other dysesthesias, night pain or disturbed sleep, and antalgic postures or movement patterns. Pain described as burning, shooting, sharp, or electric-shock-like would be more consistent with peripheral neuropathic symptoms.36 This cluster of findings was found to have a sensitivity of 90.9% and a specificity of 91.0%. A more specific response to the mechanical factors of position, load, and tension should be expected with individuals with dominant nociceptive symptoms.
Using anatomic sources: identifying the whereThe lumbar spine, SIJ, and hip are three potential anatomic sources of symptoms with the highest probability of pain generation that should be considered during the examination process in patients who have LBP. The lumbar spine is known to be the most likely primary anatomic source of the patient's symptoms, observed approximately 2/3 of the time.7,8 This should be the starting point in hypothesis testing in the context of symptom modification testing and/or interventions directed to this region. Symptom modification in the lumbar spine, in any portion of the active, passive, or passive accessory motion examination, indicates treatment as identified by symptom behavior but does not rule out distal influences from the hip or SIJ.
In the absence of lumbar symptom modification, the progression of assessment from proximal to distal allows for the most apparent differentiation of the pain generating structure, mainly if there is somatic referred pain. The lumbar spine should not reproduce symptoms before progressing distally, as SIJ dysfunction is generally identified through a process of exclusion.47 Symptom provocation tests have been well researched in this area, and the cluster described by Laslett et al.48 has been shown to have utility in both ruling out and ruling in the SIJ as the anatomic source of the pathology. Once determined as an anatomic source of pain or dysfunction, it should then be determined if the SIJ has issues of hyper or hypomobility. Modifiable symptoms of SIJ hypermobility may be determined through a positive active straight leg raise test49 or if function can be significantly improved by generating internal force closure via muscular contraction50 or externally with an SIJ belt.51 Modifiable symptoms of SIJ hypomobility may be determined via symptom provocation during compressive tests such as the SIJ compression test48 and the FABER test.52
Hip pathology, particularly hypomobility, is a commonly identified potential anatomic source or contributing factor in patients with LBP.53–56 Generating a hypothesis based on the presence of hip hypomobility and testing the hypothesis by providing an intervention that should improve hip hypomobility and then reassessing the impact of the improved mobility on the patient's primary symptomatic complaint may provide an easy access point to understand the role of hip hypomobility as a primary or secondary driver of the patient's symptomatic complaint.57
In the context of determining where the above examples are meant to illustrate one possibility of how the patient's story may unfold. In the context of identifying where, working proximally to distally while first ruling out symptom provocation from the lumbar spine, followed by the SIJ and hip, may provide the most pragmatic way to funnel the primary location that needs the most attention at any given time. In the context of the test-treat-retest model of assessing within and between-session change, it has been our clinical experience that it is easier to get something moving than it is to make something more stable or stronger. With hypermobile presentations, ensuring that distal hypomobility is not contributing to proximal hypermobility before initiating interventions to improve stability may be the most pragmatic approach.
Using mechanical inputs: identifying the howIn this context, we are defining the how as the primary mechanical input that significantly changes the patient's primary complaint at any given time if we are addressing nociceptive and/or peripheral neuropathic mechanisms as the why. Independent of the cause of the symptomatic output, the ability to change the patient's primary symptoms through mechanical input suggests that the patient's primary complaint at that time is not primarily nociplastically mediated. In this context, the patient should respond favorably to modifications in load, position, and/or tension. Each mechanism should be considered as an aspect of an overlapping Venn diagram, as all three sources of symptoms may occur at the same time, and the interaction of these factors can all be considered in the context of the movement system.
The influence of load may first be considered mechanically. Load increases through compression or decreases through distraction. Load also may be regarded as relative to changes to the patient's position (standing versus sitting versus laying down), relative to the presence and absence of muscular contractions, or relative to the compressive forces generated by tissues on a stretch. The influence of load is considered in the context of the “wher” as it relates to known effects on anatomic structures.
The impact of the position may be explored by identifying how the patient responds to modifications in end-range positions, posture, mid-range, and end range motion, and mid-range and end range repetitive motion. Exploring these variables and determining their impact on the patient's primary symptomatic complaint should help the clinician identify how to modify and change mechanical interventions to improve the patient's primary symptomatic complaint at any given time. Inherent in this model is the understanding that changes in position cause changes in load and tension around the articular structure in question.
Tension refers to the stretch of tissues, including muscles, ligaments, capsules, and nerves. Modifications in tension frequently overlap with changes in position. This may be explored by muscle length testing, neurodynamic testing, and ligamentous stress tests. For example, if a patient experiences leg symptoms in a slump test position, which is alleviated by returning the lumbar spine to extension, it may indicate tension sensitivity of the nerve, or it may indicate a positional sensitivity to lumbar flexion. Conversely, a patient with leg symptoms in a slump, whose symptoms worsen in lumbar extension, is likely to have a positional sensitivity due to a neuroforaminal interface issue rather than adverse neural tension. Thoughtful use of change in position or tension will allow the sensitivity to be discerned during testing.
If the patient responds to load, tension, and position, then their primary symptoms are probabilistically dominated by nociceptive or peripheral neuropathic mechanisms. If the patient's primary symptoms do not respond to load, tension, or position, or stop responding to modification of these variables, their primary symptoms are probabilistically being driven by a nociplastic mechanism. If this is true, they should respond more favorably to interventions meant to address more centrally mediated changes if the patient does not have red flag findings.
Putting it all togetherThe performance of the physical exam to rule out serious pathology and contributing factors by region is based on the patient's primary complaint at any given time. This complaint may be related to alterations in body structures and function, activity limitations, and participation restrictions while considering the environment and personal factors that are attained while taking the patient's history. The application of this hypothesis testing strategy may not be possible in patients who have centrally mediated symptoms.26 Focusing on symptoms in this subgroup of patients may erode the therapeutic alliance by creating unrealistic patient expectations regarding physical therapy interventions and/or result in the creation of hypervigilant behaviors. Dominant centrally mediated pain must be identified early in the process of hypothesis generation.
The pain diagram may be useful in hypothesis generation by allowing the patient to provide a visual representation of symptoms: local, proximal to distal, or global, including symptoms that may represent a centrally mediated source of symptoms or red flags that need to be ruled out. Chronic widespread pain, defined as ≥ 20% of coverage of the surface area of a pain diagram, has been shown to be correlated to higher anxiety scores, psycho-social stressors, significant life events, and the use of a greater number of pain management strategies.58 Very early in the exam process, this tool may be valuable in helping the clinician decide to evaluate and treat, evaluate and refer, or refer the patient to a more appropriate practitioner to rule out conditions indicated by findings that may represent red flags.
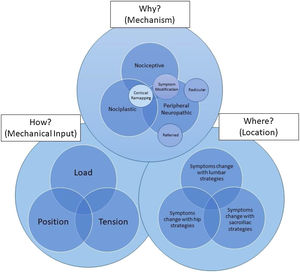
To apply this model, the clinician must first understand the potential dominant mechanisms of why the patient has symptoms at any given time (nociceptive versus peripheral neuropathic versus nociplastic), where the primary symptoms or primary contributing factor is located (lumbar spine versus SIJ versus hip), and how the patient's primary complaint may be modifiable through changes in load, tension, and position (Fig. 1). The clinician must also understand that if the mechanism (why) of the primary generator of symptoms is related to nociceptive and/or peripheral neuropathic mechanisms, a symptom modification approach is appropriate. Additionally, the clinician must understand that a different approach is required that does not focus on the patient's primary complaint if the why is nociplastically mediated. Once the clinician understands the why, where, and how they should be able to first apply these concepts in series by reflecting on action but eventually realize that these three concepts occur simultaneously with the ability to reflect in action. This is an iterative process that flows and changes based on these variables within and between treatment sessions, guided by a test-treat-retest model of care. Continuous hypothesis testing and re-assessment of the patient's response are used probabilistically to determine the most important variables to consider at any given time.
LimitationsAs a theoretical approach, the above theory stands on the same ground as other untested and unproven clinical reasoning approaches and tools.59–62 Although the current method has sought to include the concepts of reasoning and probability to eliminate some of the challenges related to the use of several current paradigms, it is not meant to imply that this approach is the only approach to the problem. While we feel that the utilization of this theoretical model should improve academic performance, clinical performance, and patient outcomes, future clinical research is needed to support these claims.
ConclusionThe theoretical model that we are proposing may help to conceptually integrate the many concepts and challenges related to the teaching and clinical application of clinical reasoning in patients with LBP. A better understanding of what these concepts are and how they are related through the proposed model may help to improve the clinical conversation, academic application of clinical reasoning, and clinical outcomes.
Conflicts of interestThe authors report that they have no conflicts of interest.