Migraine is a primary headache with high levels of associated disability that can be related to a variety of symptoms and comorbidities. The role of physical therapy in the management of migraine is largely unknown. Therefore, the aim of this review is to highlight and critically discuss the current literature and evidence for physical therapy interventions in individuals with migraines.
MethodsA narrative review of the literature was performed.
ResultsPhysical therapists assessing and treating patients with migraine should focus on two primary aspects: (1) musculoskeletal dysfunctions, and (2) vestibular symptoms/postural control impairment. Signs and symptoms of musculoskeletal and/or vestibular dysfunctions are prevalent among individuals with migraines and different disability levels can be observed depending on the presence of aura or increment of the migraine attacks.
ConclusionA proper physical examination and interview of the patients will lead to a tailored treatment plan. The primary aim regarding musculoskeletal dysfunctions is to reduce pain and sensitization, and physical therapy interventions may include a combination of manual therapy, exercise therapy, and education. The aim regarding postural control impairment is to optimize function and reduce vestibular symptoms, and interventions should include balance exercises and vestibular rehabilitation. However, consistent evidence of benefits is still lacking due to the lack of and therefore need for tailored and pragmatic clinical trials with high methodological quality.
Migraine is a primary headache disorder and among all neurologic diseases, it is considered the first cause of disability in people under 50 years of age.1 Despite its prevalence, affecting 14.4% of the population worldwide;2 and high burden, representing a major public health problem in several countries,3 migraine is still generally under-diagnosed and under-treated.4
Up to 25% of patients with migraine have their attacks accompanied by aura: transitory neurologic symptoms, including visual and/or language disturbance, and sensory or motor symptoms.5 Furthermore, around 2.5% of individuals with migraine are considered to have a chronic condition, defined as presenting with more than 15 headache days within a month.6 Subclassifications of the disease (migraine with and without aura and chronic migraine) occur clinically at various severity levels and coexist with numerous comorbidities. For this reason, several studies encourage a multidisciplinary and tailored treatment approach for these patients.7–12
Physical therapy can be considered to support and complement the pharmacological management of migraine.10,12 However, this recommendation is not well disseminated among health professionals, when compared to the management of other headache types, such as cervicogenic and tension type headaches. Evidence that physical therapy could play an important role in the treatment of migraine has been growing and it is suggested that there are currently two main approaches patients could benefit from: (1) addressing musculoskeletal dysfunctions, especially in the craniocervical region; and (2) addressing postural control impairment and vestibular symptoms. Therefore, the aim of this paper is to highlight and critically discuss the specific role of physical therapy in the management of individuals with migraine and its symptoms/comorbidities.
Musculoskeletal dysfunctionsAccording to the literature, up to 80% of patients with migraine report neck pain to some extent during the migraine-circle.13,14 The prevalence of neck pain is even greater than the prevalence of nausea, which is considered part of the migraine diagnostic criteria.15–17 The presence of neck pain is related to a worse clinical presentation of the migraine18 and it may even reduce the response to pharmacologic treatment, therefore reinforcing the migraine burden.17,19 Furthermore, the perception of tension in the neck muscles is related to stronger migraine attacks, shorter interictal phases and possibly even persistence of headache.20
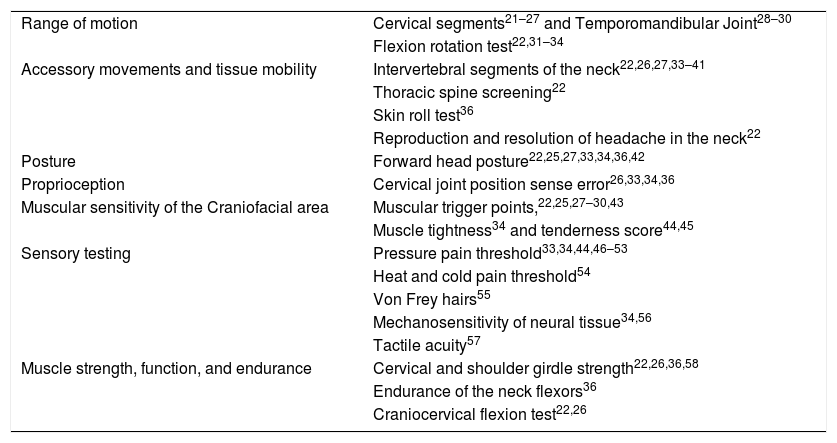
Numerous methods for identifying musculoskeletal dysfunctions are used in migraine research. These include methods that require complex equipment such as electromyography or ultrasonography as well as tests, which can be performed as a routine procedure in the clinical practice of a physical therapist. Up to 20 different clinical musculoskeletal tests have previously been described to investigate musculoskeletal differences between individuals with migraine and controls (Table 1).
Musculoskeletal tests for patients with migraine.
| Range of motion | Cervical segments21–27 and Temporomandibular Joint28–30 |
| Flexion rotation test22,31–34 | |
| Accessory movements and tissue mobility | Intervertebral segments of the neck22,26,27,33–41 |
| Thoracic spine screening22 | |
| Skin roll test36 | |
| Reproduction and resolution of headache in the neck22 | |
| Posture | Forward head posture22,25,27,33,34,36,42 |
| Proprioception | Cervical joint position sense error26,33,34,36 |
| Muscular sensitivity of the Craniofacial area | Muscular trigger points,22,25,27–30,43 |
| Muscle tightness34 and tenderness score44,45 | |
| Sensory testing | Pressure pain threshold33,34,44,46–53 |
| Heat and cold pain threshold54 | |
| Von Frey hairs55 | |
| Mechanosensitivity of neural tissue34,56 | |
| Tactile acuity57 | |
| Muscle strength, function, and endurance | Cervical and shoulder girdle strength22,26,36,58 |
| Endurance of the neck flexors36 | |
| Craniocervical flexion test22,26 |
In an international consensus-based approach, at least 11 tests were suggested as a standard for the physical examination of musculoskeletal dysfunctions in patients with headache.59 It was demonstrated that 93% of patients with migraine had at least three of these 11 musculoskeletal dysfunctions, which usually included a greater prevalence of trigger points, reduced cervical range of motion (ROM), more frequently positive cervical flexion-rotation test, more pain provocation and resolution on sustained manual palpation, and lower cervical extension strength when compared to healthy controls.22 The performance of these 11 tests takes less than 30min, and can therefore be easily integrated into a first appointment. Seven of the tests have acceptable inter-rater reliability.60
A recent meta-analysis showed that differences between patients with migraine and controls was found for cervical ROM, flexion rotation test, pressure pain thresholds, and forward head posture (standing position).61 Moreover, cervical and thoracic spine joint play and trigger point assessment are also frequently performed, using diverse techniques. Based on the recommendations from the meta-analysis for the use of physical therapy for patients with migraine, and reliability of the various tests, the forward head posture, trigger point palpation and manual palpation of the upper spine can be seen as the most practical tests to detect dysfunctions in the cervical region of patients with migraine.60,61
Patients with migraine also exhibit signs of allodynia and sensitization particularly in the facial, occipital, and cervical region. This is seen clinically when normally innocuous stimuli are painful, an observation which occurs in two thirds of these patients.62 This sensitization can be quantified using von Frey hair monofilaments testing.55 Chaves et al.54 showed that patients with migraine had hyperalgesia as measured by heat and cold pain thresholds as well as by cutaneous allodynia, and suggest this as a sign of central sensitization. Individuals with migraines generally show lower thresholds than healthy controls.63 Pain thresholds measured by using elements of quantitative sensory testing are lower in the ictal then in the interictal phase.64 A meta-analysis found that pressure pain thresholds are lower in patients with migraines for the head and neck region but not for the rest of the body.53 These findings suggest local somatosensory alterations in pain processing of patients with migraine. However, patients with migraine also had higher pain ratings to cold and electrical stimuli in non-local sites additionally indicating central alterations. Because these thresholds change during the migraine cycle, Peng and May63 classified migraine as a periodic sensory dysregulation originating from the brain.
Furthermore, pain thresholds for the temporalis and sternocleidomastoid muscles and the mastoid process were significantly lower in patients with migraine than in controls,38,40,41,44,47 demonstrating a sensitized nervous system. The same muscles present with significantly more trigger points, indicating musculoskeletal dysfunctions associated with sensitization.22,27,43 Additional areas with pain thresholds that differ in patients with migraine are over the supraorbital, median, ulnar, and radial nerves, over the trapezius, temporalis, suboccipital, anterior scalene, and levator scapulae muscles, and over various craniocervical areas.28,37–40,46,47
In addition to a greater prevalence of neck pain, patients with migraine also show a higher prevalence of temporomandibular disorders (TMD).65 The presence of TMD can be considered a risk factor for an increased frequency of headache and therefore the development of chronic migraine.66
Furthermore, it has been observed that individuals with migraine show lower strength of the neck flexors and extensors,58 but not strength of the shoulder girdle.22 These findings suggest differences not only compared to healthy controls but also compared to patients with cervicogenic headache. Interestingly, the reduction of neck extension peak force, is related to higher migraine frequency and intensity67; and women who have more active trigger points show an altered pattern of neck muscle activation during craniocervical contraction.68 Also, patients with migraine showed a higher antagonist activation of the splenius capitis muscle during cervical flexion.58 Electromyography studies of the neck muscles have shown that patients with migraine have an imbalance in the cervical muscles during force production and activity. Patients with migraine show a decrement in cervical flexors strength and a lower extensor/flexor strength ratio during voluntary contraction. Additionally, the craniocervical flexion test revealed differences in muscle performance between patients with migraine and healthy controls at a 30mmHg pressure.69
Plausible mechanismsThere is ongoing scientific debate about the role the neck plays in migraine. Neck pain is more common in migraine during the preictal phase and also during the attack.13,70 It is still not clear whether neck pain is part of the migraine prodromal symptoms and therefore part of the attack or if it acts as a trigger of the attack.14 It might also serve as an aggravating and/or perpetuating pain factors. Alternately, neck pain could be referred pain as a result of central sensitization in the trigeminocervical complex. These bidirectional neural connections might facilitate the transition from acute to chronic migraine.62 Various studies support this view by reporting more severe neck involvement and musculoskeletal findings in patients with chronic migraine compared to those with episodic migraine and healthy controls.31 It is therefore recommended to include the neck in the examination protocol.
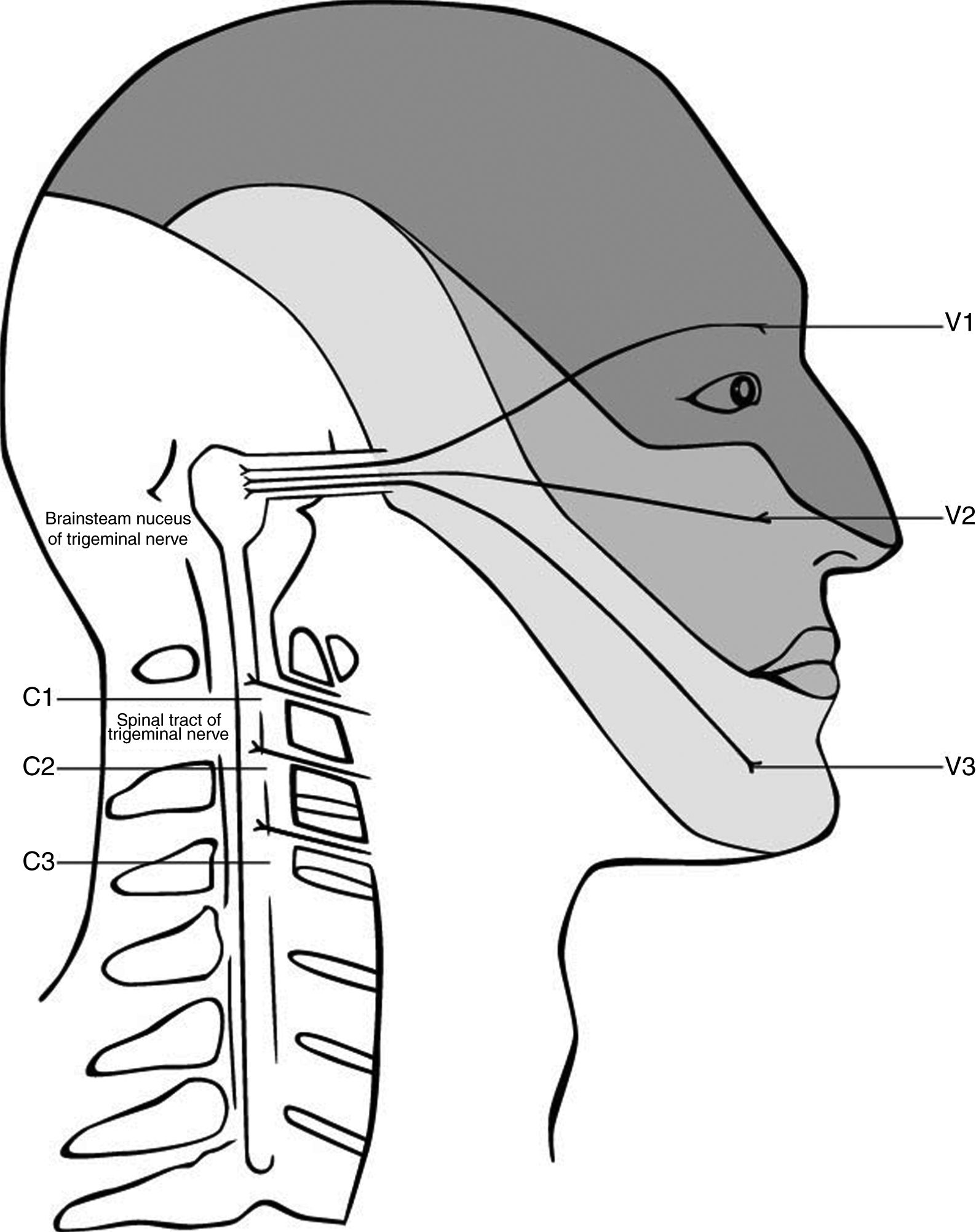
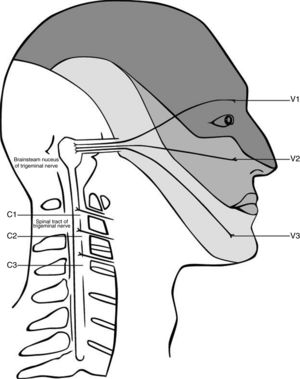
The anatomy and possible mechanism is based on the trigeminocervical complex, with convergence of nerves that innervate the head and the cervical spine (Fig. 1). Animal studies showed that afferents from the trigeminal nerve and the upper cervical spine converge into the brain stem.62,70–72 The afferents of the upper three spinal nerves pass the dorsal horn and synapse onto the spinothalamic tract. In the trigeminocervical nucleus, afferents from C1, C2, and C3 converge onto second order neurons that also receive afferents of the first division of the trigeminal nerve via the trigeminal nerve spinal tract.73 This convergence has the potential of misperceived sources of pain in higher centers74 and can therefore explain referred pain in both directions. Thus, the activation in the trigeminocervical nucleus may have its origin in either cervical or trigeminal areas.
Convergence of the cervical and trigeminal nerves in the brainstem. Adapted from Haldeman and Dagenais, 2001.79
Painful stimuli converge with second-order neurons in the caudal part of the brain stem in the pars caudalis of the nucleus spinalis n. trigemini.75 Sensitization in this trigeminocervical complex may contribute to headaches. Accordingly, a dysfunction in the musculoskeletal area that has afferents into the trigeminocervical complex, may reinforce sensitization and thereby facilitate chronicity. As the dominant side of the headache does not influence the site of sensitivity to pressure over nervous tissue, it seems like a generalized sensitization through the neck might influence the headache.37 Another confirmation of that hypothesis is the response of migraine to local therapies such as the injection of local anesthesia and/or cortisol in the region of the greater occipital nerve, which mainly consist of fibers of C2.76,77
Nociceptive afferents coming from the temporomandibular joint might also sensitize the trigeminal system. Facial stimulation has an enhanced response in brainstem trigeminal neurons after a chemical stimulation of the intracranial dura, indicating a connection between these pain processing areas.78
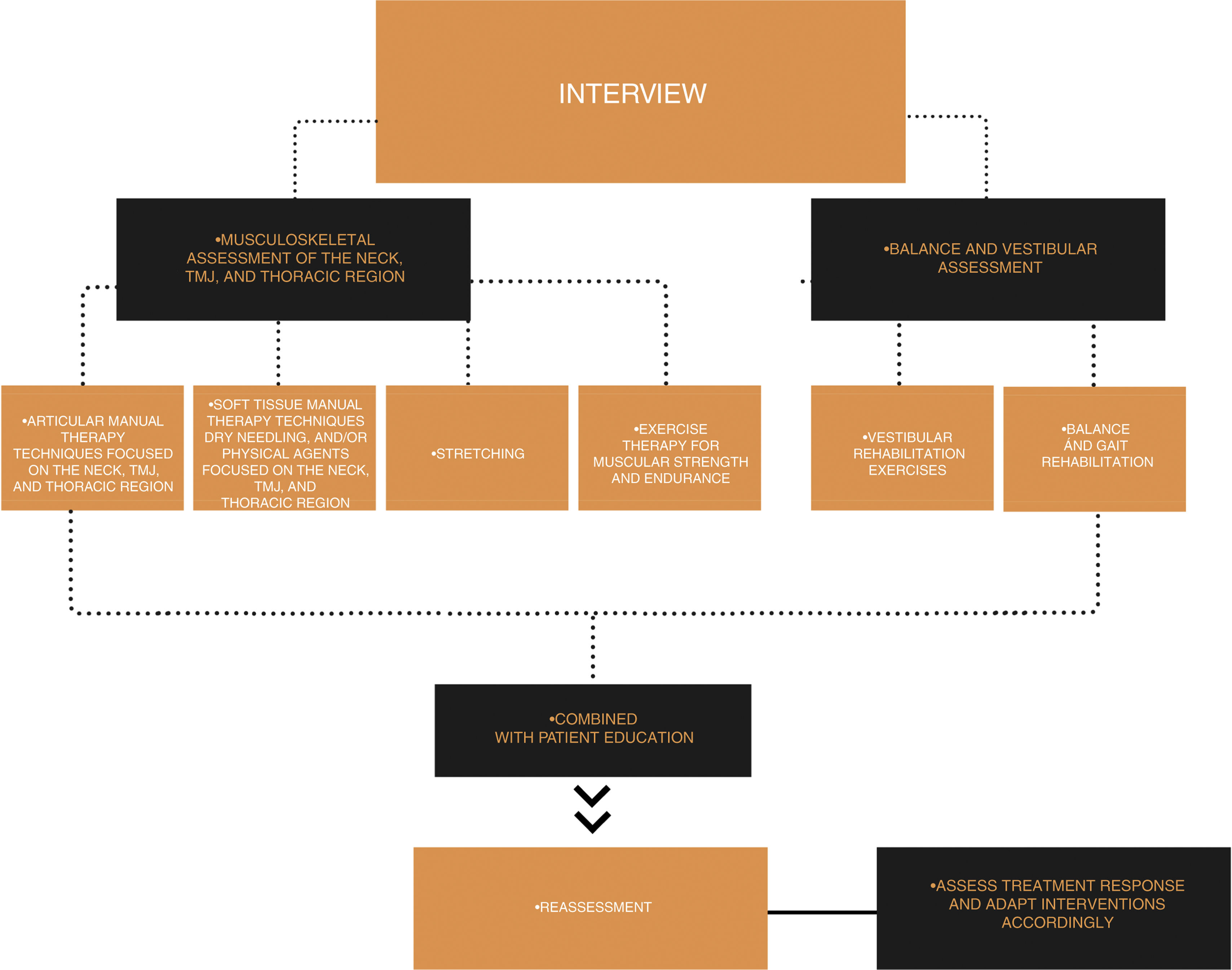
Physical Therapy approach for musculoskeletal pain and dysfunctionThe treatment recommended in guidelines for migraine to reduce the frequency of headache days is mainly pharmacological. Preventive medication designed to reduce the attack frequency is often taken for months or years and most medications have side effects/adverse events.80,81 International guidelines recommend only few non-pharmacological treatment approaches, these include relaxation, behavioral therapy, and aerobic exercise.82–85 These approaches can and should be part of physical therapy. However, physical therapy could offer more extensive treatment options. So why should we think of migraine as a disease for physical therapy interventions? The essence of physical therapy is movement. It is about maintaining the function of the neuromusculoskeletal system and the restoration, enhancement, and preservation of movement and physical function when impaired or threatened.84,86
According to the International Classification of Headache Disorders,15 headache diagnosis relies mostly on the patient interview, which might not be in itself enough to recommend physical therapy, without a formal evaluation of potential musculoskeletal dysfunctions. Thus, physical therapy examination should be based on information from the patient interview and the practitioners’ clinical reasoning process. In this process it is important to have a patient-centered approach in which the patient is not only treated with a hands-on approach but also educated and counseled in a manner providing the opportunity to affect not only pain in the neck and migraine attacks but also self-efficacy to improve quality of life. According to the results of the interview and the examination, the treatment should be planned individually, and it should involve – depending on the patient - manual therapy, exercises, and education.
A recent study suggests that manual treatment of trigger points and stretching of the sternocleidomastoid and upper trapezius muscles – especially when combined with treatment of suboccipital muscles significantly reduce the impact and disability of migraine.87 A recent review summarized that manual treatment of trigger points may reduce the frequency, intensity, and duration of attacks although some studies did not meet high methodological quality standards.88 A pilot study published after this review shows that up to four physical therapy sessions provided every 2 weeks are enough to show positive results.87 To maintain the effects of trigger point treatment, it is recommended to combine manual treatment with exercises to strengthen the neck and scapular musculature and to emphasize an internal locus of control.89
Passive treatment methods should always be incorporated within a more comprehensive approach focusing on active form of therapy and patient participation. A tailored treatment approach, based on physical findings, is recommended. For example, if the examination reveals reduced mobility or pain in the cervical or thoracic region the patient could be taught specific exercises; if dysfunction or weakness of the scapular musculature is identified, strengthening exercises are used, and finally, in the presence of trigger points, stretching of the relevant muscles may be a useful option.
It has been shown that neck pain can be reduced after eight weeks of cervical strength training in patients with chronic neck pain.90 In addition, scapula positioning exercise can lead to a significant reduction in chronic neck pain.91 Because of the high prevalence of neck pain in individuals with migraines, cervical and thoracic strength training may therefore help many of those patients. It might also help in reducing the painful input into the trigeminocervical complex, which may prompt more migraine events, or at least to reduce the burden of neck pain for those patients.
Studies using standard protocols for manual therapy interventions for the spine show either no or little effect on migraine frequency, but demonstrate nevertheless that pressure pain thresholds increase, clinically relevant changes occur, and patients’ satisfaction is reinforced.92 Taken all together it can be recommended to combine exercises (eg, strengthening in cervical extension and flexion and nerve tissue mobilization) and manual therapy (eg, cervical mobilization and suboccipital muscle tone reduction) to help reduce pain and disability.93 Furthermore, interventions focused on the temporomandibular joint and related muscles should be used when indicated, and aerobic exercises especially of high intensity are recommended.94–96
As migraine is long-lasting and therefore considered a chronic pain condition, a primary treatment focus should be on education and behavior modifications. Studies of various quality and different approaches suggest that patient education can improve migraine frequency, disability, and quality of life.97 Topics related to migraine physiology, identification and modification of migraine triggers, self-management strategies, emotional coping, and medication safety are suggested. Behavioral education on relaxation and diet are also recommended.97
Knowledge about pain physiology can reassure patients that migraine is not a life-threatening condition and therefore reduce the fear of pain and also the pain itself. In studies of patients with migraines, education is typically part of a multimodal approach including behavioral therapy.97 It should address the understanding of the symptoms from a neurophysiological point of view to reduce fear and catastrophizing and emphasize an activity-based approach.98 This is of particular interest because 53% of patients with migraine report kinesiophobia.99 Physical therapists can be part of a multimodal treatment and can help patients to improve their self-efficacy and thus achieve a better quality of life and reduce the level of pain. However, education does not seem to help with depressive symptoms,97 anxiety, and stress, which is often present in these patients. Therefore, psychological treatment must additionally be included in the comprehensive management of migraine.100,101
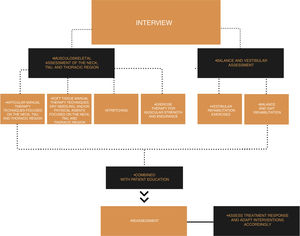
A summary of the suggested physical therapy interventions for patients with migraine is shown in Fig. 2.
Vestibular symptoms and postural control changesThe presence of vestibular symptoms is also considered inherent to the migraine condition.102 This includes self-reported dizziness, defined as the disturbance of spatial orientation, vertigo, a false sensation of self-motion or visual surround motion, and/or postural symptoms, defined as unsteadiness and instability while upright.103 It is estimated that the prevalence of these symptoms ranges between 12% and 85%.104–107 A higher prevalence is associated with the presence of aura, higher pain intensity, and greater migraine frequency.106,107 Efforts from the International Classification of Headache Disorders (ICHD-3) have been centred towards a sub classification of these patients with a Migrainous Vertigo diagnosis. However, due to the need of validation and the common overlap with other migraine diagnoses and with other disorders, this classification remains in the ICHD-3 Appendix.15,104
Nevertheless, the presence of vestibular symptoms should be thoroughly assessed and managed, such symptoms can be present not just during, but also between the migraine attacks.106,107 Vestibular symptoms are associated with greater migraine-related disability, higher depression levels,105 and also moderate-to-severe handicap due to the dizziness itself – especially affecting the physical and functional domains.107
Patients with migraine, compared to headache-free individuals,108–119 and other headache diagnoses,118,119 can also present deficits in postural control, including impairment of balance in quiet standing. Interestingly, balance impairment is not influenced by the presence of dizziness or vertigo,109,110,112,115,118–123 but by the presence of aura, chronicity of the headache attacks,112,113 or presence of an attack.124 However, aura and chronic migraine do not seem to have any influence on the performance of mobility tasks such as walking, negotiating obstacles, or performing sit-to-stand tasks, which are equally reduced in all migraine diagnosis groups when compared to controls.115,121 Progression of the impairment in mobility and static tasks are observed in this group of patients over a one-year-period.114
Furthermore, patients with migraine show a reduction of the limits of stability, which encompass the ability to control the displacement of the Center of Pressure without changing the base of support.121 Reduction of stability limits leads to a reduction in anticipatory postural adjustments, and perturbations beyond these limits are related to a lack of balance control and greater risk of falls.125 Indeed, it has been verified that early postural control deterioration in patients with migraine is related to a greater self-report of falls126,127 and fear of falls, especially when aura and chronicity are present.127 This aspect is often underestimated by patients and clinicians, because migraine per se is a very disabling condition; and patients between the ages of 20–55 years old are less susceptible to injury-related falls in comparison to older adults, which present with other comorbidities such as osteoporosis, arthrosis, or deficits in protective reflexes.127
Plausible mechanismsThe presence of the above-mentioned signs and symptoms can be due to sensory information mismatch from the proprioceptive, vestibular, and visual systems.128–130 Gazing a visual motion stimulus, for example, can trigger an illusory self-movement sensation, and visual afferences will not match the vestibular or proprioceptive cues, which are predicted based on previous experiences.131 The conflict between expected and perceived cues among these three systems can arise from an underlying dysfunction in peripheral structures including the labyrinthic, visual, proprioceptive, and exteroceptive afferents. Moreover, it can be triggered by malfunctioning of central nervous system structures as well, including the brain stem, cerebellum, inner ear, basal ganglia, and cortical hemispheres.132,133
It is estimated that around 58% of patients with migraine present abnormalities in the function of the vestibular system based on vestibular tests,108,111,116,134–137 and the dysfunction prevalence is similar among patients with and without self-report of vestibular symptoms.116,136,137 For this reason, it remains unclear why some patients with migraine present these symptoms and others do not.106,136,138
Additionally, a delayed development of visual motion processing and anomaly in orientation perception139–144 are verified in individuals with migraines, culminating in functional movement alterations that can be observed from an early age.145 Other etiology hypotheses include brain hyperexcitability,139,143,146–149 overlap among the trigeminal and vestibular pathways,115,116,118,122,150structural and functional dysfunction of the visual network,143,151,152 and presence of ischemic-like lesions in the labyrinth, brainstem,153,154 and cerebellum.117,148,155–164
Physical Therapy approach of vestibular symptoms and balance dysfunctionWhile interventions targeting musculoskeletal pain in individuals with migraine are increasingly discussed in the literature, to the best of our knowledge, very few reports address the benefits of balance and vestibular rehabilitation in this population.
Vestibular rehabilitation consists of a set of exercises involving eye and head movements performed during activities such as sit-to-stand, walking, bending over, and throwing a ball. These progressive exercises were originally developed by Cawthorne and Cooksey with the aim to target gaze stability and visual motion sensitivity and improve vestibular habituation.165 According to a guideline from the American Physical Therapy Association, there is strong evidence towards the effectiveness of this approach in patients with vestibular hypofunction compared to sham or no intervention for improvement in symptom intensity, quality of life, function, and psychological outcomes. Exercises should be performed under supervision as well as at home at least 3 times a day. No consensus exists whether certain vestibular exercises are more effective than others.166
Even though the presence of migraine impacts negatively on the vestibular rehabilitation outcomes and delays the treatment success, this approach is recommended for this population.126,167–171 Improvements after vestibular rehabilitation could be observed in balance oucomes,126,168,171 decrement of self-report of falls,126 and also headache attenuation.172 However, conclusive evidence regarding the efficacy of vestibular rehabilitation in migraine is still missing, due the lack of prospective, blinded, randomized controlled studies.173 Other interventions including balance, gait, global endurance, and strength training, which when combined, can improve fall rates, balance, and mobility in older adults,174–177 even long term.178 Because migraine can be recognized as a condition related to early balance deterioration, leading to similar rates of falls as older adults,127,179 the above mentioned treatment strategies could potentially be beneficial. However, to date, these interventions have not been studied in a migraine population.
Future directions in researchDespite all the knowledge we have about the prevalence of neck pain in patients with migraine, there are only few studies, and no randomized controlled trials of high quality on the physical treatment of migraine. Most studies investigate multimodal or multidisciplinary treatments94,180 or show little effects.87,92 That may be one reason why physical therapy examination and treatment for the neck is not yet considered in the international guidelines for migraine.
Previous clinical trials focusing on physical therapy for patients with migraine have major limitations. One potential reason why available studies only show minor cervical impairments in individuals with migraine when compared to controls, might be patients selection. Future studies should aim to distinguish between patients with episodic, highly frequent, and chronic migraine. Furthermore, patients should be sub-grouped according to musculoskeletal findings to evaluate whether there are patients that could benefit more from physical therapy. Another reason why the effects of physical therapy might be underestimated is the choice of outcome measures, with the traditional primary outcomes being headache frequency and intensity. Future studies should also report self-reported ability to cope with the burden of headache and secondary outcomes like disability, quality of life, and non-headache measures.181 Furthermore, clinical intervention may need to be applied in a pragmatic manner, tailored to each patient as opposed to a standardized treatment protocol.
As the latest review on therapeutic patient education revealed that more studies with high methodological quality are needed,97 studies regarding the amount, the best method of administration, and the most important content of an education program are high priority. Also, the optimal manner to integrate education with hands-on physical therapy approaches must be addressed. Accordingly, our recommendation is to treat musculoskeletal impairments and dysfunctions relying on sound clinical reasoning and a patient centered approach.
Despite one study that reported a progression in balance deficits over a one year-period in a migraine population,114 little is known about the clinical relevance of these deficits and if they persist or continue to get worse with aging. Many studies have also demonstrated balance deficits in the absence of dizziness,109,110,112,115,118–123 and that vestibular dysfunction can be present independent of vestibular symptoms.116,136,137 Furthermore, special attention should be employed in patients with a high headache frequency and the presence of aura.
Based on the current evidence, it seems that there is a severity continuum among individuals with migraine, suggesting that vestibular migraine should not be considered as a separate entity, with a distinct pathophysiology.107,116,134 Future investigations should be performed to better understand the comorbidity between migraine, balance disorders, and vestibular symptoms. In addition, the mechanisms and susceptibility of migraine to these comorbidities should be clarified.
Finally, there is an emerging need for studies testing the effectiveness of physical therapy interventions related to balance, mobility, and vestibular symptoms in this population. Are vestibular rehabilitation and other balance training exercises beneficial to these patients either as a treatment or for prevention? Which are the factors that influence outcomes? Which patients are most likely to benefit from such treatment? Answers to these questions, based on high-quality clinical trials and systematic reviews, would certainly move science towards better care and reduction of the migraine burden.
ConclusionsIndividuals with migraines seek non-pharmacologic treatments to reduce headache frequency and improve quality of life.182 Physical therapy could potentially reduce musculoskeletal impairments related to neck pain prevalent in this population. Furthermore, vestibular symptoms and postural control impairment should also be addressed when present, to optimize function and reduce the presence and severity of vestibular symptoms.
Conflicts of interestThe authors declare no conflicts of interest.
AcknowledgementsFAPESP foundation (process number: 2018/12024-5).
Migraine research foundation, DMKG.