The combination of interval training and resistance training has showed interesting results in chronic heart failure patients, corroborating the benefits of physiological adaptations of both protocols.
ObjectiveTo evaluate the effect of the combination of interval training and resistance training program when compared to interval training alone and/or without intervention group on cardiorespiratory fitness in patients with chronic heart failure.
MethodsWe search MEDLINE via PubMed, ScienceDirect, Sportdiscus, BIREME and Scielo, from their inception to December 2018. Were included both randomized and non-randomized controlled trials comparing the effect of combined training, interval training alone and/or WI group on VO2peak (expressed in ml/kg/min), in people with chronic heart failure patients. The meta-analysis was conducted via Review Manager v 5.3 software, using random effect model.
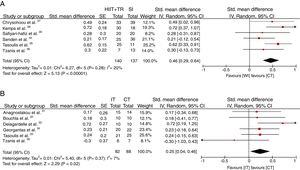
ResultsTen articles were selected (nine randomized controlled trial), involving 401 participants. Six studies compared combined training with interval training and six studies compared combined training with the without intervention group. Eighty percent of the trials presented moderate risk of bias and twenty percent low risk of bias. Data showed significant difference and major increase in VO2peak in favor to combined training group compared to interval training group (SMD=0.25; CI=0.04–0.46) and without intervention group (SMD=0.46; CI=0.29–0.64), respectively.
ConclusionThe combination of interval training and resistance training showed more effective in increasing cardiorespiratory fitness in patients with heart failure than interval training alone and non-exercise therapy. However, further studies should be conducted to increase the understanding of this combined training method.
Chronic heart failure (CHF) is a complex clinical syndrome, characterized by cardiac dysfunction, caused by structural and/or functional irregularity,1 in which there is an inadequate volume of blood ejection to supply the metabolic needs of all organs and tissues of the body.1,2 This process may result in reduced cardiac output and elevated intracardiac pressure at rest and/or during stressful situations.2 CHF is recognized as a new epidemic in several countries.1,3 In the last decades, there has been an increase in the number of adults and elderly population diagnosed with CHF.4,5 This is mainly due to changes in lifestyle, such as excess weight and its comorbidities, as well as the increase in sedentary behavior in all age groups.6,7 Furthermore, CHF is one of the main reasons for hospitalization and high hospital costs, which results in high rates of mortality and morbidity of the patients.1,3,4
After diagnosis, the reduction of the level of physical activity contributes even more to increasing the intolerance to exercise and the symptoms of the syndrome0, which may affect the peripheral vasodilator response, leading to greater endothelial dysfunction and to a progressive decrease in functional capacity.1,2 Thus, Guyda et al.8 reported that the reduction in peak oxygen uptake (VO2peak) is highly associated with morbidity and mortality in cardiac patients. VO2peak is considered an important marker for the longevity and quality of life of patients.7,8 Thus, non-pharmacological treatment in the form of physical exercise is considered an indispensable therapeutic tool, which proves to be safe and inexpensive.1,9 It is well reported that regular exercise increases muscle function and cardiorespiratory fitness (CRF), as well promotes greater body efficiency in using oxygen.10
Therefore, different methods and protocols of exercises have been researched. Interval training (IT) and resistance training (RT) have shown interesting protocols to promote benefits for patients with CHF.11–13 IT is characterized in the literature by short intermittent periods of vigorous activity, alternated with recovery intervals or low-intensity exercise.14 This protocol has been proposed in traditional settings of supervised cardiac rehabilitation.12 IT is effective training with limited risks and promotes potential health benefits in patients with CHF.15,16 Among the principles that IT follows, we can mention sprint interval training (SIT) and high-intensity interval training (HIIT).17 SIT is composed of sprints that last between 8 and 30s at an intensity of 100% of VO2max, while HIIT is characterized by sets of efforts lasting between 60 and 240s, with intensity greater than 80% of VO2max.14,17 On the other hand, RT is a training protocol that requires muscle work against a load or resistance – this may be an external load or even body weight.18
Combining these protocols, also known as combined training or concurrent training (CT), has often been applied both in the child and adolescent population, as well as in adults and the elderly with different health conditions.19–21 In addition, CT has showed interesting results in CHF patients, corroborating the benefits of physiological adaptations of both protocols.22 In addition, it may promote additional benefits in vascular endothelium and muscle strength in comparison to IT alone, while improving aerobic parameters and exercise tolerance.23,24
However, although several studies have described the importance of exercise therapy in CHF patients, there has been no systematic review analysis of CT in scientific literature in recent years. Thus, we propose an in-depth analysis of the effects of IT plus RT protocol on CRF in patients with CHF, since physical fitness is an important component of the lifespan and quality of life for this population. Therefore, this study aimed to perform a systematic review with meta-analysis to compare the effects of interval training combined with resistance training versus interval training and the non-intervention group on cardiorespiratory fitness of chronic heart failure patients.
MethodsThe search strategies were carried out in September 2017 and updated in December 2018, based on the recommendations of the Preferred Reporting Items for Systematic Review and Meta-analyses: The PRISMA Statement.25 We also followed the Cochrane Handbook for Systematic Reviews,26 as well as the tutorial for systematic reviews of Brazilian Journal of Physical Therapy.27 This systematic review was prospectively registered on the PROSPERO database (CRD42018116071).
Types of studiesWe included both randomized and non-randomized controlled trials that included patients with chronic heart failure that made the combined training protocol resistance training plus interval training and compare with interval training alone or a without intervention control group (WI).
Types of participantsWere included human patients of both sexes diagnosed with chronic heart failure, with no other congenital heart disease, atrial fibrillation or heart transplant implants.
Types of interventionsIT is characterized when carried out by short intermittent periods of vigorous activity, alternated with recovery intervals or low-intensity exercise. RT is considered as exercise that requires muscle work against a load or resistance – this may be an external load or even body weight. Were considered an intervention of combined training when the RT and the IT were performed at the same session.
ComparisonsWe were interested in compare the protocol of combined training (RT plus IT) with two other groups: (a) without intervention group (control group, non-exercise group, wait list control, and placebo); and (b) interval training alone group.
Comparisons were designed as:
- •
Combined training vs. Without intervention group;
- •
Combined training vs. Interval training.
The primary outcome of this study was the CRF, measured by change in VO2peak expressed in ml/kg/min at baseline and post intervention.
Search methodsThe searches were conducted in five databases: MEDLINE (Medical Literature Analysis and Retrieval System online) via PubMed, ScienceDirect, Sportdiscus, BIREME (Latin American and Caribbean Center on Health Sciences Information) and Scielo. The databases were selected according to the area of knowledge and scientific relevance worldwide. Furthermore, a search was performed on the references of each study found and selected in the searches.
Thus, the descriptors were selected based on index terms via DECS (BIREME health science subject descriptors) and MESH (Medical Subject Headings – controlled vocabulary used for indexing articles in PubMed). The following descriptors were chosen in English and Portuguese: heart failure; aged; cardiorespiratory fitness; oxygen consumption; resistance training; strength training; high-intensity interval training; and sprint interval training. The descriptors were combined with the Boolean terms “AND” and “OR”. The main search strategy used in all databases was heart failure OR aged AND cardior-respiratory fitness OR oxygen consumption AND resistance training OR strength training AND high-intensity interval training OR sprint interval training, respectively. There was no temporal delimitation of the articles found.
Screening and selection processAll duplicate articles were removed. The first screening was followed by reading the titles and abstracts of original articles whose summary was available for reading, on human samples that presented chronic heart failure, interval training intervention combined with resistance training and that presented an assessment of VO2peak, before and after intervention.
Subsequently, the studies were assessed for eligibility by reading the articles in full-text. All the stages, such as selection and election of the studies, were evaluated by two researchers (FJMJ) and (ICJ) independently. The reviewers compared the studies and, in case of disagreement, a third reviewer (NL) arbitrated. Any disagreements about a study's eligibility were decided by a consensus meeting.
The following variables were recorded in a database independently by each investigator: author, publication year, average age of the sample, number of individuals in the control group, the number of individuals in the experimental group, the physical training protocols (volume, load, duration, intensity and type of exercise) and maximum oxygen consumption (mean value and standard deviation) before and after intervention.
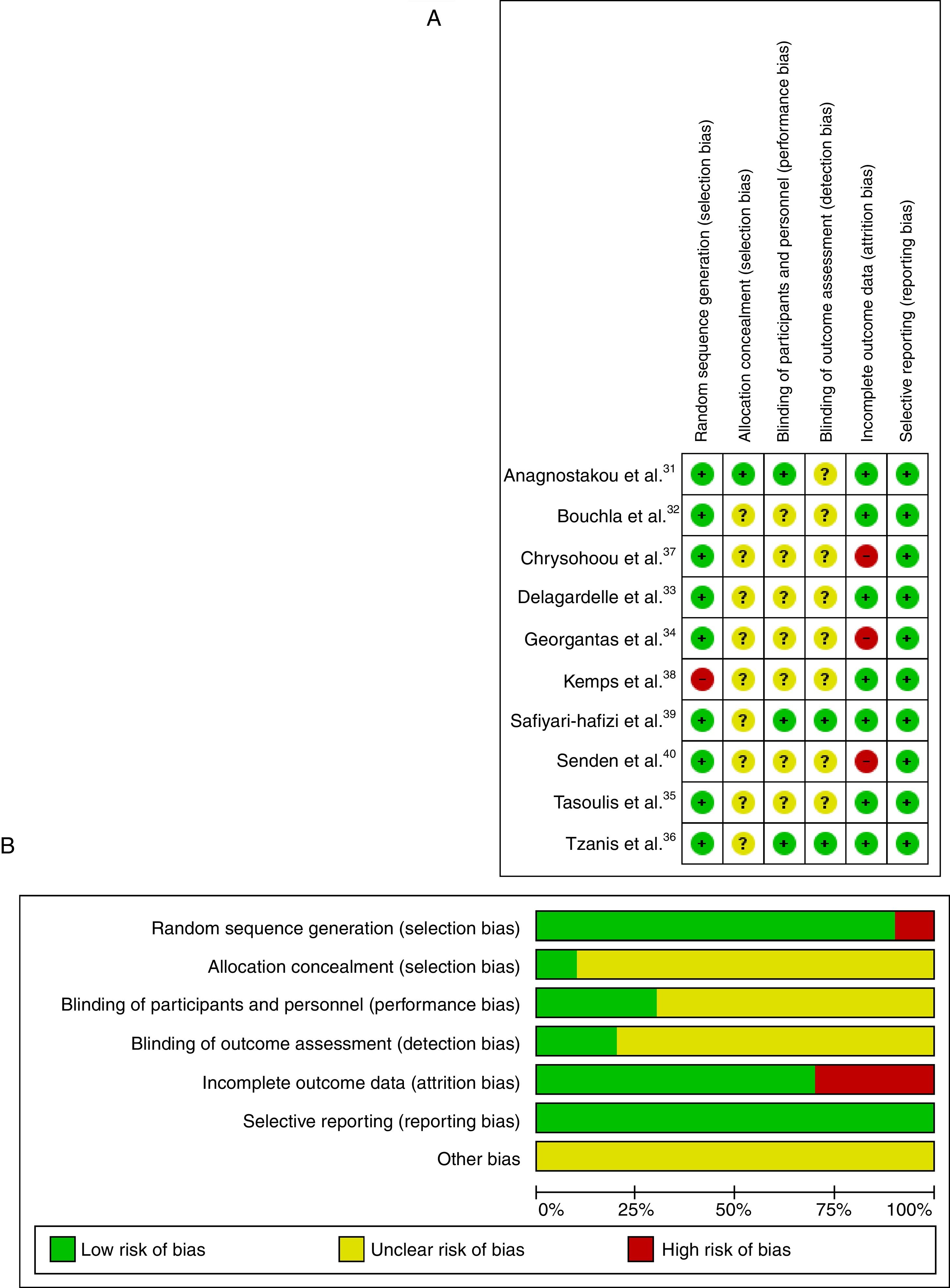
Risk of biasOnce selected, all studies were analyzed for risk of bias, following the risk of bias of the Cochrane Collaboration tool, which assesses the risk of high, low or unclear bias.26 Two reviewers (FJMJ and ICJ) applied to the scale are composed of five criteria that are important for almost experimental and experimental studies, namely: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; and (6) selective reporting, the evaluation was standardized following the Cochrane Handbook of Systematic Reviews of Interventions.26 In the event of disagreement, a disagreement shall be settled by a third arbitrator (NL).
Quality of evidenceThe quality of the evidence for each outcome was analyzed according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE),28 which consists of five items: (1) risk of bias; (2) inconsistency; (3) indirectness; (4) imprecision and (5) other considerations. The assessment was made by two reviewers (FJMJ and ICJ) and if any disagreement were found, it was discussed, if there was no agreement between the reviewers, a third reviewer (NL) arbitrated. The quality of the evidence was classified into four categories: high, moderate, low or very low, according to the information provided by the GRADE system and on the recommendations made by Balshem et al.28
Synthesis of data and statistical analysisData from the change in VO2peak from post-intervention, characteristics of patients, as well as details of training protocols were analyzed and archived in a database for tabulation. Moreover, risk of bias was also assessed and described, as well as intergroup characteristics in the pre-intervention period, such as: age, gender, preserved or reduced ejection fraction, New York Heart Association (NYHA) classification and left ventricular ejection fraction (%LVEF). In the absence of information relevant to the primary outcome of the study, correspondent authors were contacted in order to request more detail. If there was no answer from the respective authors in relation to the request, the study was excluded from the final analysis.
The meta-analysis was conducted via Review Manager v 5.3 software, using a random effects model. The effect size of treatment was obtained by means of the difference of the variation between the pre- and post-intervention period, described by standardized mean difference (SMD) and the standard error (SE) adjusted for both CT group and the IT control group and the without-intervention group (WI).29 In addition, the analysis of heterogeneity among the studies was obtained through I2 test, in which I2 of <25%, 25–50% and >50% were considered small, medium and large inconsistency, respectively.30
Sensitivity analysisSensitivity analysis was performed following two procedures: (1) including only reporting of studies with complete’ training protocols; (2) by removing the classified studies with a high risk of bias.
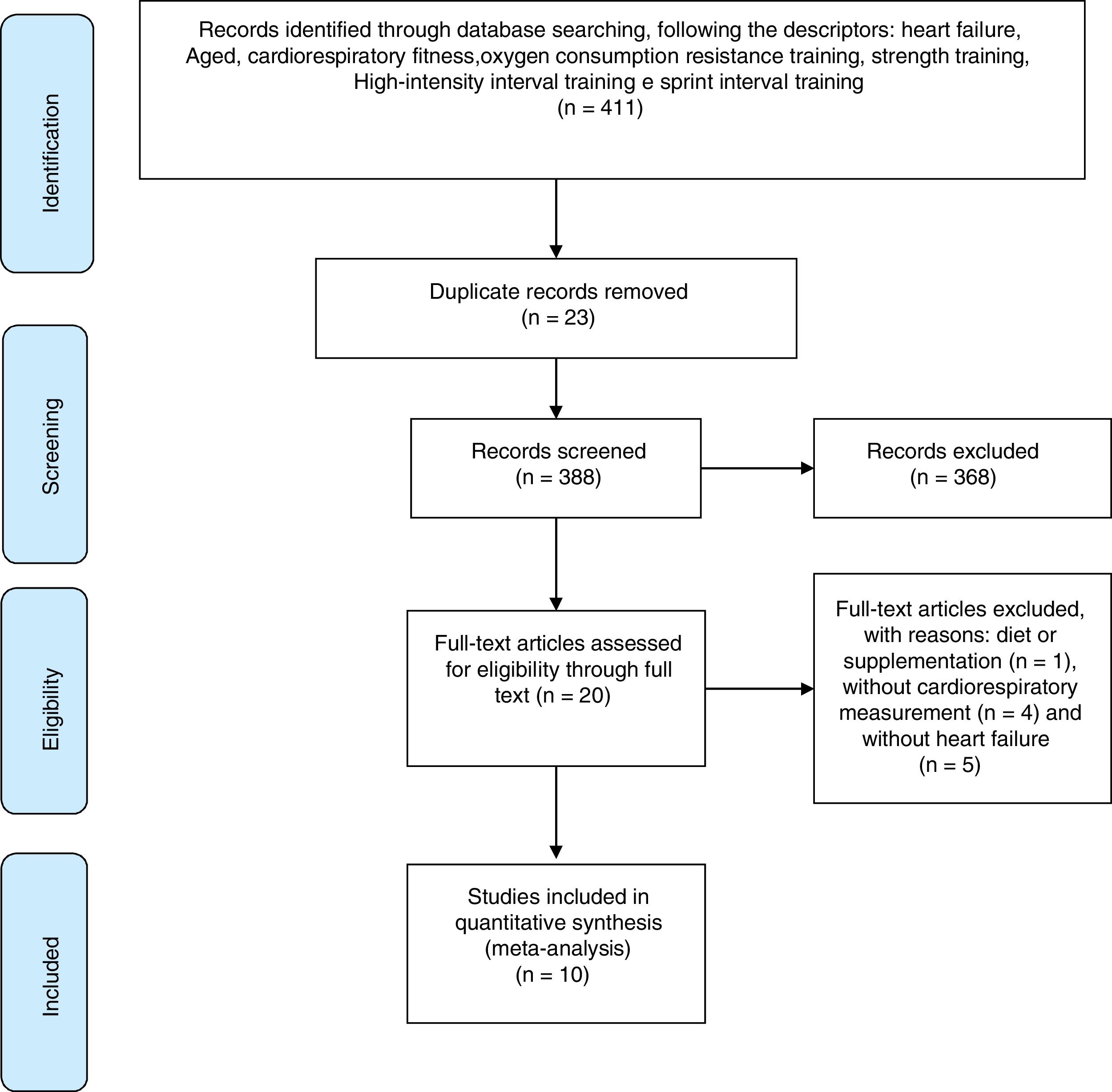
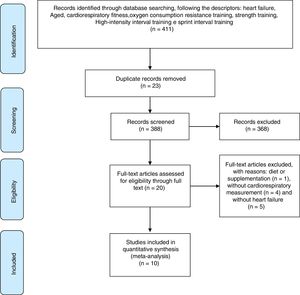
ResultsSearch resultsFirstly, 411 studies were found following a combination of descriptors. Sequentially, 23 studies were excluded by duplicate examination. Of the remaining 388 studies 368 did not meet the proposed criteria, in that 144 were not original articles, 19 studies had no abstract available, 21 studies were conducted with animal samples and 184 were without a CT protocol.
Thus, 20 studies were assessed for eligibility and, where necessary, excluded for the following reasons: one study investigated the effect of supplementation, four studies had no evaluation of CRF and five were not composed of patients diagnosed with HF. Therefore, the search was finalized with 10 selected articles. The search steps, selection and eligibility of studies are outlined in Fig. 1.
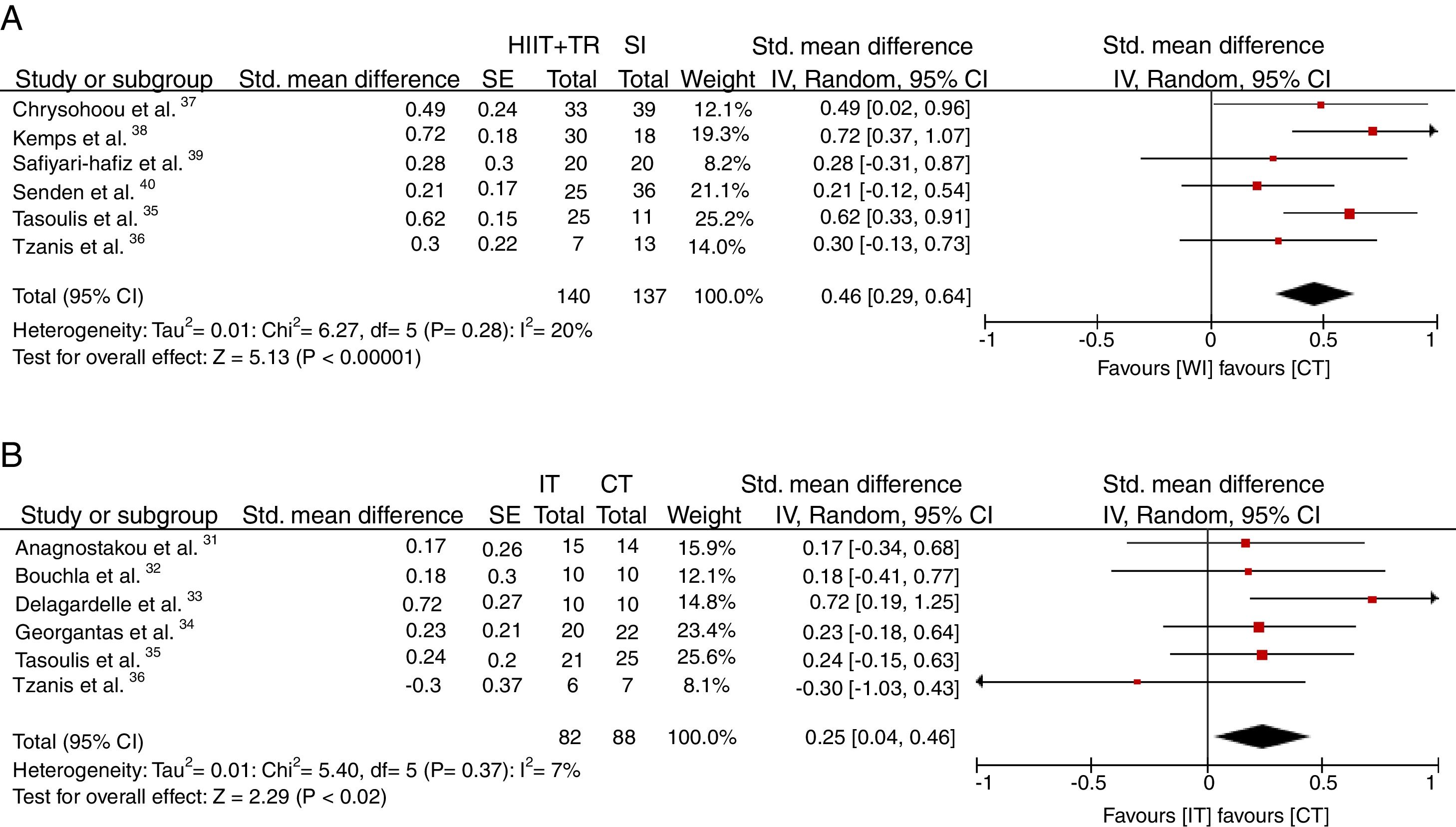
Primary features of articlesThe selected articles were published between 2002 and 2017. All studies were performed on patients with CHF. A total of 188 patients underwent CT, 81 performed only IT and 132 formed the without intervention group (WI). Only one study was non-randomized controlled trial (RCT).38 In addition, 6 studies compared CT with IT31–36 and 6 studies compared CT with the WI group.35–40
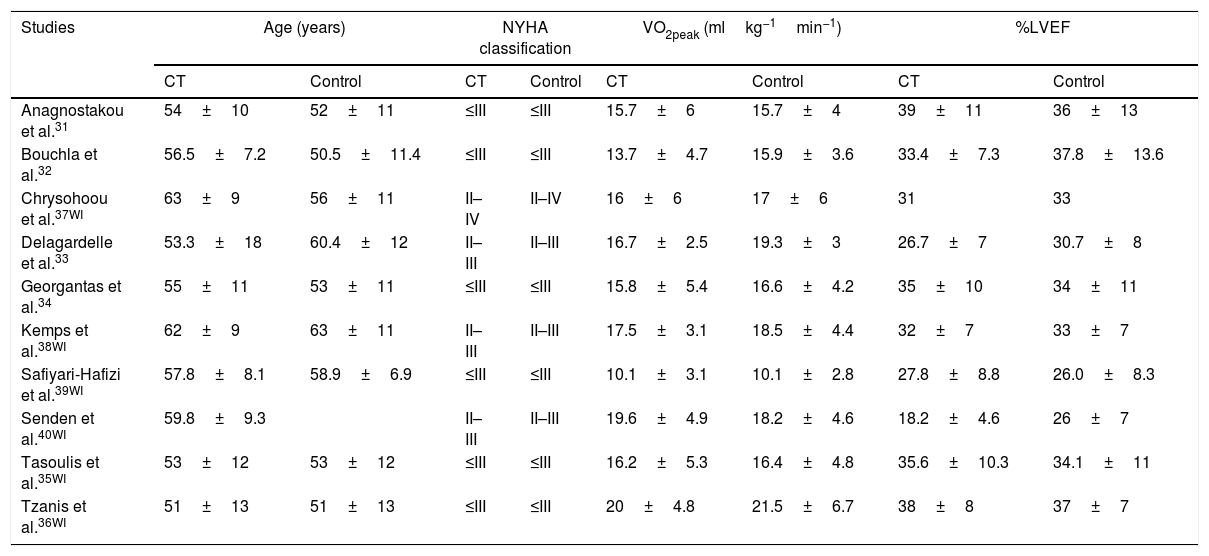
Characteristics of patientsThe patients had a mean and standard deviation age of 51±13 years36 and 63±9 years.37 In six studies, patients had NYHA functional class≤III,31–36,38–40 in three NYHA functional class between II and III,33,38,40 and a NYHA functional class between II and IV.37 All studies indicated that patients had reduced ejection fraction.31–40 The main characteristics of the samples are presented in Table 1.
Characteristics of pre-intervention groups.
| Studies | Age (years) | NYHA classification | VO2peak (mlkg−1min−1) | %LVEF | ||||
|---|---|---|---|---|---|---|---|---|
| CT | Control | CT | Control | CT | Control | CT | Control | |
| Anagnostakou et al.31 | 54±10 | 52±11 | ≤III | ≤III | 15.7±6 | 15.7±4 | 39±11 | 36±13 |
| Bouchla et al.32 | 56.5±7.2 | 50.5±11.4 | ≤III | ≤III | 13.7±4.7 | 15.9±3.6 | 33.4±7.3 | 37.8±13.6 |
| Chrysohoou et al.37WI | 63±9 | 56±11 | II–IV | II–IV | 16±6 | 17±6 | 31 | 33 |
| Delagardelle et al.33 | 53.3±18 | 60.4±12 | II–III | II–III | 16.7±2.5 | 19.3±3 | 26.7±7 | 30.7±8 |
| Georgantas et al.34 | 55±11 | 53±11 | ≤III | ≤III | 15.8±5.4 | 16.6±4.2 | 35±10 | 34±11 |
| Kemps et al.38WI | 62±9 | 63±11 | II–III | II–III | 17.5±3.1 | 18.5±4.4 | 32±7 | 33±7 |
| Safiyari-Hafizi et al.39WI | 57.8±8.1 | 58.9±6.9 | ≤III | ≤III | 10.1±3.1 | 10.1±2.8 | 27.8±8.8 | 26.0±8.3 |
| Senden et al.40WI | 59.8±9.3 | II–III | II–III | 19.6±4.9 | 18.2±4.6 | 18.2±4.6 | 26±7 | |
| Tasoulis et al.35WI | 53±12 | 53±12 | ≤III | ≤III | 16.2±5.3 | 16.4±4.8 | 35.6±10.3 | 34.1±11 |
| Tzanis et al.36WI | 51±13 | 51±13 | ≤III | ≤III | 20±4.8 | 21.5±6.7 | 38±8 | 37±7 |
Note: NYHA, New York Heart Association; %LVEF, left ventricular ejection fraction; WI, without intervention.
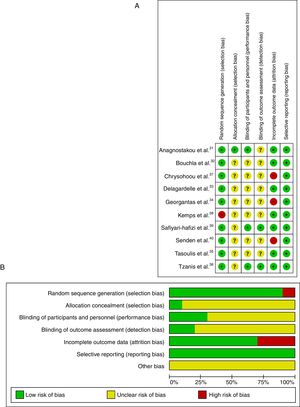
The risk of bias in the included articles was considered high, as shown in Fig. 2A. The main risk of bias was related to the allocation concealment.
Risk of bias across studiesThe risk of bias across studies is shown in Fig. 2B. Random sequence generation and incomplete outcome data were the most prevalent risk of bias across the eligible trials. However, allocation concealment, blinding of participants/personnel and blinding of outcome assessment were unclear in 80% of the studies.
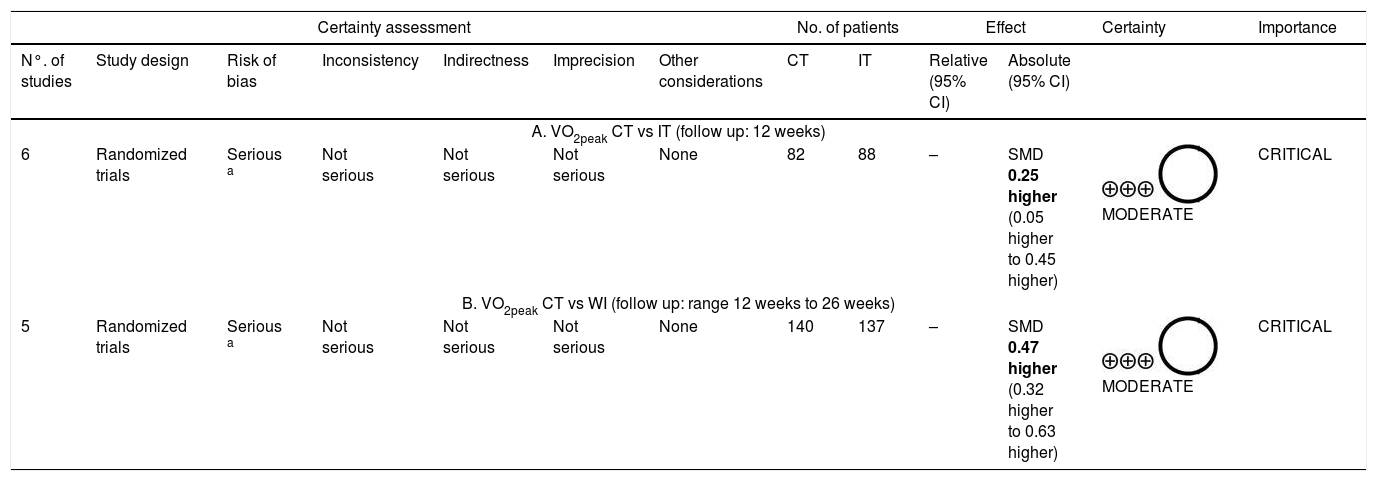
Quality of evidenceStudy quality was assessed using the GRADE system, which indicated moderate quality with serious or not serious methodological problems. Only one non-RCT38 was not included in the analysis of evidence for CT vs WI comparison. Quality assessment is shown in Table 2.
Quality of evidence (GRADE) between CT versus IT (A), and CT versus without intervention (B) for VO2peak.
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N°. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | CT | IT | Relative (95% CI) | Absolute (95% CI) | ||
| A. VO2peak CT vs IT (follow up: 12 weeks) | ||||||||||||
| 6 | Randomized trials | Serious a | Not serious | Not serious | Not serious | None | 82 | 88 | – | SMD 0.25 higher (0.05 higher to 0.45 higher) | MODERATE | CRITICAL |
| B. VO2peak CT vs WI (follow up: range 12 weeks to 26 weeks) | ||||||||||||
| 5 | Randomized trials | Serious a | Not serious | Not serious | Not serious | None | 140 | 137 | – | SMD 0.47 higher (0.32 higher to 0.63 higher) | MODERATE | CRITICAL |
Note: CI, confidence interval; SMD, standardized mean difference; CT, combined training; IT, interval training; WI, without intervention.
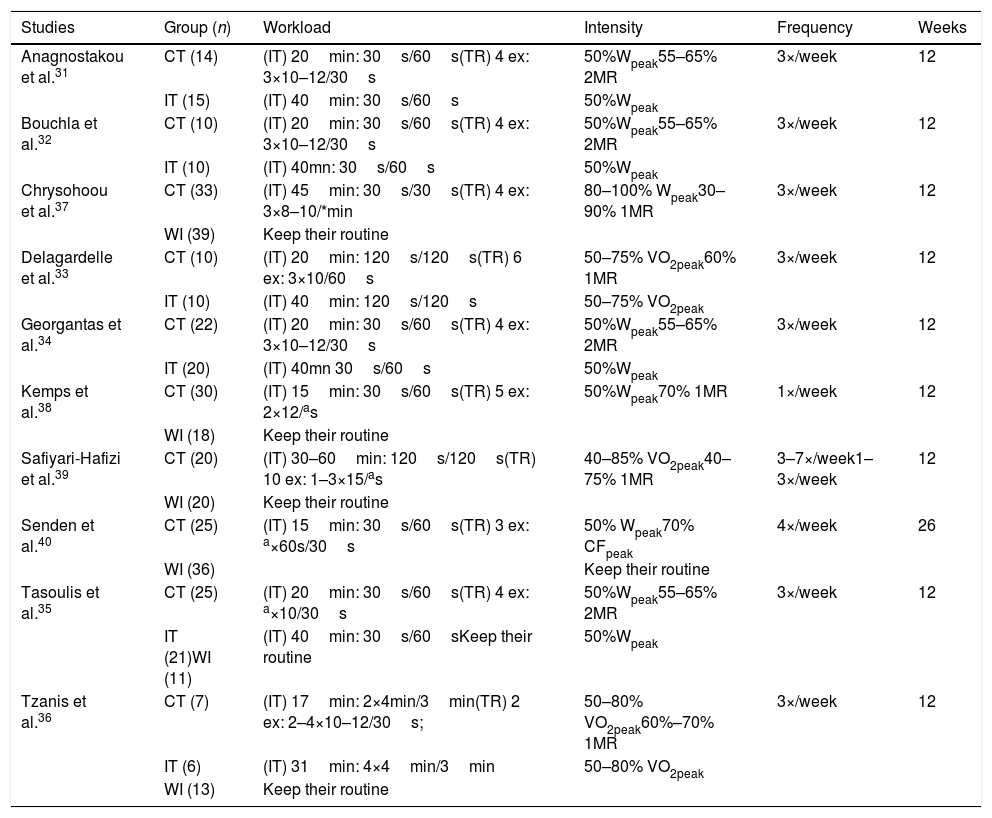
The CT protocols used in the studies showed similarity, in which 90% performed interventions lasting 12 weeks, except for one study that performed 26 weeks of intervention.40 All IT protocols were performed on a stationary bicycle, and the 30s/60s (effort/rest) ratio was predominant, with total duration of sessions last between 15 and 45min,31,32,34,35,37,38,40 except for two studies that used 120s/120s33,39 and a study that used 240s/180s.36 The intensities of the interval exercise ranged from 50%Wpeak,31,32,34,35,38,40 80–100% Wpeak37 and 40–80%VO2peak.33,36,39 Regarding TR, the majority of the studies used four exercises31,32,34,37,35 with the exception of those who used ten,39 six,33 five,38 three40 or two36 exercises per session. The load intensity ranged from 60 to 80% of one maximum repetition (MR)33,36–40 and 55–65% of two MR.31,32,34,35 Details of training groups and protocols are highlighted in Table 3.
Description of intervention groups and protocols.
| Studies | Group (n) | Workload | Intensity | Frequency | Weeks |
|---|---|---|---|---|---|
| Anagnostakou et al.31 | CT (14) | (IT) 20min: 30s/60s(TR) 4 ex: 3×10–12/30s | 50%Wpeak55–65% 2MR | 3×/week | 12 |
| IT (15) | (IT) 40min: 30s/60s | 50%Wpeak | |||
| Bouchla et al.32 | CT (10) | (IT) 20min: 30s/60s(TR) 4 ex: 3×10–12/30s | 50%Wpeak55–65% 2MR | 3×/week | 12 |
| IT (10) | (IT) 40mn: 30s/60s | 50%Wpeak | |||
| Chrysohoou et al.37 | CT (33) | (IT) 45min: 30s/30s(TR) 4 ex: 3×8–10/*min | 80–100% Wpeak30–90% 1MR | 3×/week | 12 |
| WI (39) | Keep their routine | ||||
| Delagardelle et al.33 | CT (10) | (IT) 20min: 120s/120s(TR) 6 ex: 3×10/60s | 50–75% VO2peak60% 1MR | 3×/week | 12 |
| IT (10) | (IT) 40min: 120s/120s | 50–75% VO2peak | |||
| Georgantas et al.34 | CT (22) | (IT) 20min: 30s/60s(TR) 4 ex: 3×10–12/30s | 50%Wpeak55–65% 2MR | 3×/week | 12 |
| IT (20) | (IT) 40mn 30s/60s | 50%Wpeak | |||
| Kemps et al.38 | CT (30) | (IT) 15min: 30s/60s(TR) 5 ex: 2×12/as | 50%Wpeak70% 1MR | 1×/week | 12 |
| WI (18) | Keep their routine | ||||
| Safiyari-Hafizi et al.39 | CT (20) | (IT) 30–60min: 120s/120s(TR) 10 ex: 1–3×15/as | 40–85% VO2peak40–75% 1MR | 3–7×/week1–3×/week | 12 |
| WI (20) | Keep their routine | ||||
| Senden et al.40 | CT (25) | (IT) 15min: 30s/60s(TR) 3 ex: a×60s/30s | 50% Wpeak70% CFpeak | 4×/week | 26 |
| WI (36) | Keep their routine | ||||
| Tasoulis et al.35 | CT (25) | (IT) 20min: 30s/60s(TR) 4 ex: a×10/30s | 50%Wpeak55–65% 2MR | 3×/week | 12 |
| IT (21)WI (11) | (IT) 40min: 30s/60sKeep their routine | 50%Wpeak | |||
| Tzanis et al.36 | CT (7) | (IT) 17min: 2×4min/3min(TR) 2 ex: 2–4×10–12/30s; | 50–80% VO2peak60%–70% 1MR | 3×/week | 12 |
| IT (6) | (IT) 31min: 4×4min/3min | 50–80% VO2peak | |||
| WI (13) | Keep their routine |
The measurement of CRF was expressed by VO2peak (ml/kg min). All studies assessed the CRF using symptom-limited incremental exercise protocol on an electromagnetically braked cycle ergometer, with direct gas monitoring to assess VO2peak.
The standardized mean difference in the relative VO2peak was 0.47ml/kg/min [CI 95%: (0.32–0.63) p<0.00001; I2=20%] compared to the WI group (Fig. 3A), and 0.25ml/kg/min [CI 95%: (0.05–0.45) p<0.02; I2=7%] in favor of the CT compared with the IT protocol (Fig. 3B).
However, there was no significant change in the result of the analysis when including only studies with a full description of exercise protocols, or when the studies had a high risk of methodological bias.
DiscussionThe results of this review indicate that interventions using the CT method may promote more relevant adaptations to CRF compared to IT and SI groups. These findings were observed in other studies using CT. These studies described the increase in maximum oxygen consumption in patients with hypertension,41 overweight adults42,43 and patients with cardiovascular disease.21
As previously reported, the reduction in VO2peak is closely related to morbidity and mortality in cardiac patients.1,8 It represents an important marker for longevity and quality of life in those patients1,8,44. Guidelines for CHF patients indicate that regular exercise is a safe and low-cost form of treatment, and it has great importance to the success of the treatment0,0. It is strongly recommended that at least 30min of aerobic activities at moderate to vigorous intensity should be done between 3 and 5 times per week, along with resistance exercises at least twice per week at moderate intensity (60–70% 1MR).44
IT has been recommended as a non-pharmacological treatment for elderly with cardiovascular conditions.8,12,44 This demonstrates a stronger effect on aerobic capacity, the remodeling of left ventricular reversion, endothelial function and quality of life compared to continuous aerobic exercise.16,44 Furthermore, IT revealed a direct relationship between the intensity of physical training and the magnitude of improvement in aerobic fitness with low risks for exercise-related adverse events0. On the other hand, RT is considered an important method for the improvement and maintenance of functional capacity, strength and muscle power in the elderly and patients with CHF.9,45,46 Strength exercises combined with aerobic activity have been shown to promote better results related to body composition, muscle strength and CRF in individuals with obstructive coronary disease when compared to aerobic training alone.47,48
Studies suggest that CT increases muscle function and aerobic capacity, by promoting greater efficiency of the body to use oxygen,2,33 being effective for maintaining physical fitness and functional capacity0. It is worth mentioning the importance of maintaining physical fitness with aging as a relevant factor for the reduction in the development of risk factors, such as the risk of chronic cardiometabolic diseases, type 2 diabetes, metabolic syndrome, cardiovascular disease and obesity, and the reduction in functional capacity and activities of daily living.18,45
In this review, all the studies presented equivalence of volumes and training between the intervention groups CT and IT, which may strengthen the findings. It was observed that the CT protocols were considerably homogeneous, and in addition, 70% of the studies presented the SIT protocol.31,32,34,35,37,38,40 Therefore, in 50% of the studies, the CT corresponded to the duration of approximately 40min per session, using four exercises of RT at 55–65% of 2RM and 60–80% of 1RM, while for the IT protocols, mostly used 30s/60s ratio, with a moderate intensity of 50%Wpico and a frequency of 3 times per week, respectively, with satisfactory and consistent results.31,32,34,35,37,38,40 This pattern of volume, intensity and training frequency corroborates with the recommendations for the practice of physical exercises in patients with CHF.44
The implementation of CT (IT plus RT) may maximize treatment time, an aspect that has been considered as an important factor when choosing an intervention.24,46,47 The characteristic of this training method reinforces the growing interest in this type of exercise, which is becoming an interesting strategy in terms of increasing aerobic capacity, as well as a relevant tool for the treatment of CHF patients.49 The results of the present meta-analysis may indicate a new path for future research and may well help future studies to conduct better methodologically delineated interventions in this type of protocol.
This study has some limitations. First, most of the studies found do not comply with the methodological recommendations for secret allocation and controlled clinical trials, as well as presenting reduced samples. And second, none of the studies dealt with nutritional control, which together with physical exercise is an important aspect of non-pharmacological treatment.
In conclusion, the results suggest that CT may be considered as another efficient option, demonstrating the development of relevant adaptations to aerobic fitness for the treatment of CHF patients. Therefore, CT may be capable of promoting superior VO2peak benefits, although IT also provides benefits in VO2peak compared to the non-intervention group. However, further studies should be conducted to broaden the understanding of this training method, exploring different exercise volumes and intensities, as well as comparing with other CT methods.
Financial supportThis work was funded by CNPq and CAPES.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Araucária, Santander Universities 2018 Grants Program and Fundação para a Ciência e Tecnologia (FCT/UID/DTP/00617/2013).