To address the need for a better treatment of chronic whiplash associated disorders (WAD), a contemporary neuroscience approach can be proposed.
ObjectiveTo examine the effectiveness of a contemporary neuroscience approach, comprising pain neuroscience education, stress management, and cognition-targeted exercise therapy versus conventional physical therapy for reducing disability (primary outcome measure) and improving quality of life and reducing pain, central sensitization, and psychological problems (secondary outcome measures) in people with chronic WAD.
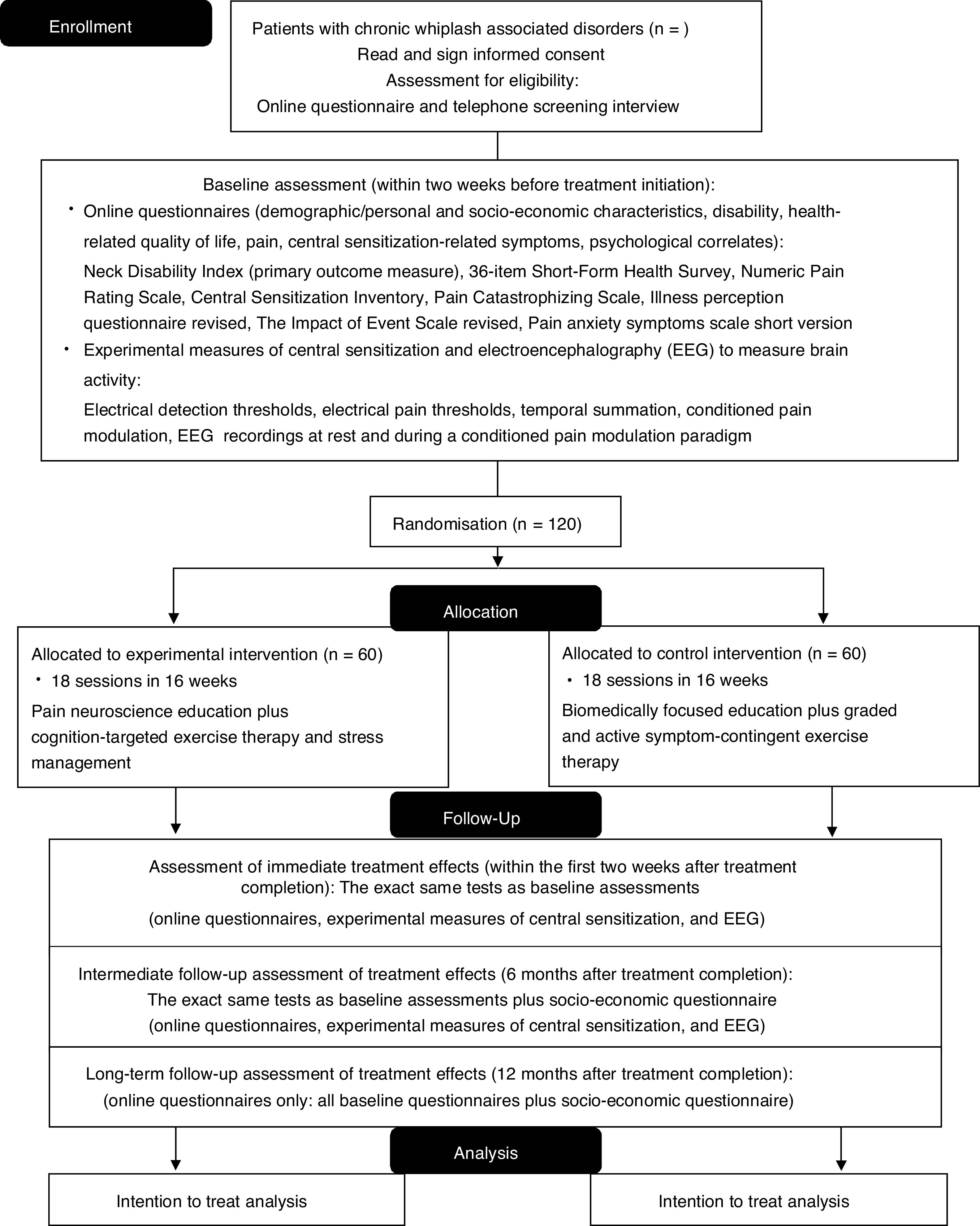
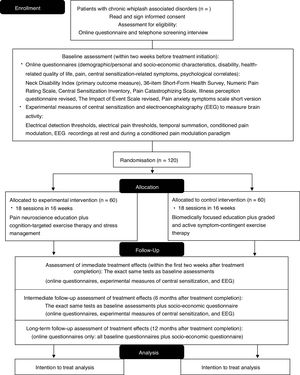
MethodsThe study is a multi-center, two-arm randomized, controlled trial with 1-year follow-up and will be performed in two university-based and one regional hospital. People with chronic WAD (n=120) will be recruited. The experimental group will receive pain neuroscience education followed by cognition-targeted exercise therapy, and stress management. The control group will receive biomedically focused education followed by graded and active exercise therapy focusing on muscle endurance, strength, and flexibility, and ergonomic principles. The treatment will have a duration of 16 weeks. Functional status (Neck Disability Index) is the primary outcome measure. Secondary outcome measures include quality of life, pain, central sensitization, and psychological and socio-economic factors. In addition, electroencephalography will measure brain activity at rest and during a conditioned pain modulation paradigm. Assessments will take place at baseline, immediately post-treatment and at 6 and 12 months follow-up.
ConclusionsThis study will examine whether a contemporary neuroscience approach is superior over conventional physical therapy for improving functioning, quality of life, and reducing pain, central sensitization, and psychological problems in people with chronic WAD.
Whiplash injury is one of the most common traffic-related injuries.1 Approximately 50% of people who encounter a whiplash injury will continue to experience ongoing disability and pain 1 year after the injury.2 Poor treatment responses and treatment of patients with chronic whiplash associated disorders (WAD) remain a challenge for health care professionals.3–6
In chronic WAD, substantial evidence supports the presence of central sensitization.7 Central sensitization encompasses various related dysfunctions of the central nervous system, all contributing to increased responsiveness to a variety of stimuli.8 In many people with chronic WAD, central sensitization and the resulting exaggerated pain and disability dominate the clinical picture over the cervical dysfunctions. It is known that people will move differently when they are in pain9 and the brain does not always require nociception – anticipation of pain suffices – to change its way of controlling body movement.10 Therefore, it is warranted to reconceptualize pain (e.g. pain does not per se result from tissue damage) before moving to more active treatment approaches.11,12 This could be addressed by reducing the threat value of pain by means of pain neuroscience education (PNE).13
PNE is effective for improving maladaptive pain beliefs and decreasing disability and pain in various chronic pain disorders,13–17 including chronic WAD.13 However, PNE is not a stand-alone treatment and the effect sizes are small to moderate, but it is a crucial first step to prepare patients for a time-contingent, cognition-targeted approach to physical activity and exercise therapy.
A dysfunctional stress response and posttraumatic stress symptoms can be present in patients with chronic WAD.18–21 Posttraumatic stress symptoms have a negative influence on the recovery and severity of WAD complaints.22 Also, pain and stress are closely interconnected23 and stress is a sustaining factor of central sensitization.24 Hence, addressing stress via a stress management intervention integrated in the treatment for chronic WAD is important in the contemporary neuroscience approach accounting for central sensitization.24
Once the patient has adopted adaptive beliefs, the next step can be taken: dynamic and functional exercise therapy. This therapy should be cognition-targeted including exercises that are performed in a time-contingent manner.25 A previous randomized controlled trial showed that PNE combined with cognition-targeted exercise therapy is superior over biomedically focused education and pain-contingent exercise therapy in patients with nonspecific chronic spinal pain.26 While few promising studies have examined a combined approach of PNE with specific exercise therapy,14,16,26,27 none focused specifically on patients with chronic WAD.
To our knowledge, this will be the first sufficiently powered randomized controlled trial to examine the effectiveness of a contemporary neuroscience approach (i.e. PNE combined with stress management and cognition-targeted exercise therapy) versus conventional physical therapy for various important outcome measures in people with chronic WAD. The primary objective is to examine whether the contemporary neuroscience approach is more effective than conventional physical therapy in reducing disability (primary outcome measure) and improving quality of life, socio-economic factors, and illness perceptions, and decreasing pain, central sensitization, posttraumatic stress symptoms, pain catastrophizing, and pain-related fear (secondary outcome measures) in people with chronic WAD.
MethodsTrial design and settingA multi-center, randomized controlled trial, will be performed in the University Hospital Brussels and University Hospital Ghent and a regional hospital (Sint-Jozefkliniek Campus Bornem). The trial is designed as a parallel group, two-arm, superiority trial with a 1:1 allocation ratio. The study protocol is designed following the standard protocol items for randomized interventional trials (SPIRIT)28 and trial results will be reported according to the CONSORT guidelines.29
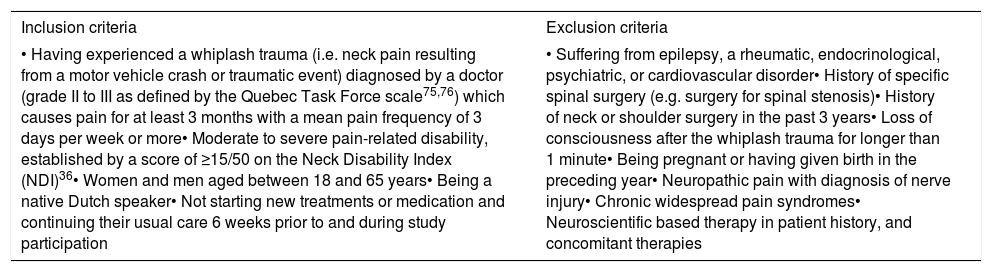
Participants and recruitment procedureOne-hundred-twenty participants with chronic WAD will be recruited. Inclusion and exclusion criteria are listed in Table 1. Participants will be recruited from the hospitals of the participating universities (UZ Brussels; UZ Ghent) and a secondary care hospital (Sint-Jozefkliniek) as well as other hospitals and universities, via social media, primary care practices, pharmacies, and publications from patients support groups. Recruitment will also take place via radio, newspapers, magazines, symposia, health insurance companies, and district health centers.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Having experienced a whiplash trauma (i.e. neck pain resulting from a motor vehicle crash or traumatic event) diagnosed by a doctor (grade II to III as defined by the Quebec Task Force scale75,76) which causes pain for at least 3 months with a mean pain frequency of 3 days per week or more• Moderate to severe pain-related disability, established by a score of ≥15/50 on the Neck Disability Index (NDI)36• Women and men aged between 18 and 65 years• Being a native Dutch speaker• Not starting new treatments or medication and continuing their usual care 6 weeks prior to and during study participation | • Suffering from epilepsy, a rheumatic, endocrinological, psychiatric, or cardiovascular disorder• History of specific spinal surgery (e.g. surgery for spinal stenosis)• History of neck or shoulder surgery in the past 3 years• Loss of consciousness after the whiplash trauma for longer than 1 minute• Being pregnant or having given birth in the preceding year• Neuropathic pain with diagnosis of nerve injury• Chronic widespread pain syndromes• Neuroscientific based therapy in patient history, and concomitant therapies |
Sample size calculation was performed with G*Power 3.1.9.2 focusing on the primary outcome measure (functional status) and primary endpoint (6 months follow-up) based on the therapy effects on functional status (difference in functional status between baseline and 6 months follow-up in a previous randomized controlled trial26 with 2 balanced treatment arms and comparable control and intervention therapies in patients with chronic spinal pain (NCT03239938)), and accounting for a 25% loss to follow-up after 6 months (based on the loss to follow-up percentage of the finished trial).26 Calculations were based on two-tailed testing with alpha=0.05 and a desired power of 0.80, with a partial eta squared=0.032, effect size=0.18, allocation ratio (N2/N1)=1, resulting in 60 patients in the experimental group and 60 patients in the control group. The total sample size is 120 people with chronic WAD. Currently, 71 patients have been included in the study and 34 patients have completed the study.
Randomization and allocation procedureParticipants are randomized in a 1:1 ratio between intervention and control arms using a stratified permuted block allocation with stratification factor being treatment center and a block size of four. Randomization lists will be prepared separately for the 3 treatment centers by an independent investigator of the Biostatistics Unit who has no other study involvement. Only 1 independent researcher, not involved in recruitment, assessments, and treatment, will perform the randomization of participants after baseline assessment, and will conceal the randomization from patients and the other researchers using opaque, closed envelopes.
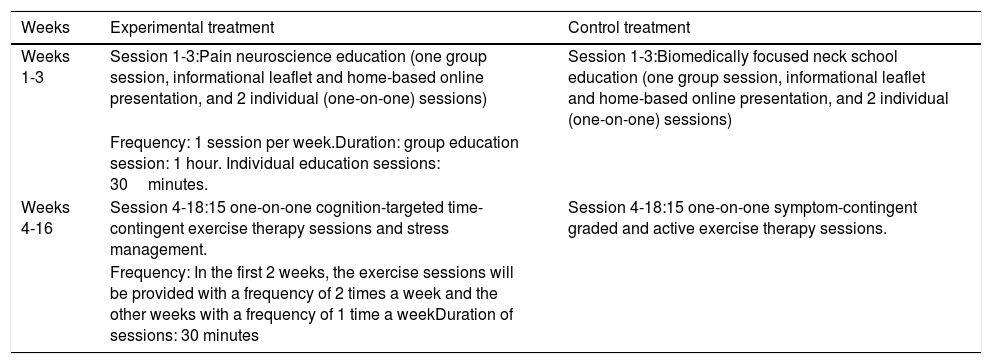
Details of the experimental and control interventionsAll participants will receive 18 therapy sessions over a period of 16 weeks (Table 2). The physical therapists will have a master's degree and will be intensively trained in performing the experimental or control treatment by physical therapists experienced in the experimental (AM, JN) or control therapy (DL, WW). Every 8 months, a refresher course will be organized.
Organization of therapeutic sessions.
| Weeks | Experimental treatment | Control treatment |
|---|---|---|
| Weeks 1-3 | Session 1-3:Pain neuroscience education (one group session, informational leaflet and home-based online presentation, and 2 individual (one-on-one) sessions) | Session 1-3:Biomedically focused neck school education (one group session, informational leaflet and home-based online presentation, and 2 individual (one-on-one) sessions) |
| Frequency: 1 session per week.Duration: group education session: 1 hour. Individual education sessions: 30minutes. | ||
| Weeks 4-16 | Session 4-18:15 one-on-one cognition-targeted time-contingent exercise therapy sessions and stress management. | Session 4-18:15 one-on-one symptom-contingent graded and active exercise therapy sessions. |
| Frequency: In the first 2 weeks, the exercise sessions will be provided with a frequency of 2 times a week and the other weeks with a frequency of 1 time a weekDuration of sessions: 30 minutes |
The contemporary neuroscience approach will comprise 3 interactive PNE sessions (1 group session followed by 2 individual sessions), and 15 sessions of cognition-targeted exercise therapy and stress management.12,25 The education covers the physiology of the nervous system in general and of the pain system in particular. The content and pictures of the educational sessions are based on current knowledge of pain science and pain education books,30,31 and the PNE content is adapted to the chronic WAD condition. Additionally, the physiology of the stress response systems and the relationship between stress and pain modulation will be explained during the PNE sessions as first educational part of the stress management.
After the PNE group session, patients will be asked to read an informational leaflet about the (neuro)physiology of pain31 and afterwards watch an online educational PowerPoint about the (neuro)physiology of pain at home. A more detailed description of how PNE will be provided is presented elsewhere.32
The cognition-targeted dynamic and functional exercise therapy will consist of 15 individual sessions. The main principles of this exercise therapy are: (1) all exercises should be performed in a time-contingent (“perform this exercise 10 times, regardless of the pain”) rather than a symptom-contingent way (“stop or adjust the exercise when it hurts”); (2) goal setting is done together with the patient, focusing on functionality instead of pain relief; (3) the therapist should continuously assess and challenge the patient's cognitions, beliefs, and perceptions about the pain and the anticipated outcome of each exercise to change maladaptive cognitions and perceptions into positive ones;25 (4) exercises should be individually tailored and gradually progress toward more feared, avoided, challenging, complex, and functional movements and activities.12
An activity form will be used to ask participants to indicate which movements and activities are feared/avoided/painful. This form will be used by the therapist to obtain a hierarchy in fearful, avoided, and/or painful movements/activities and will allow the therapist to individually tailor the exercises and create a progression in the offered exercises, movements, and activities. Communication techniques are crucial and will be aligned with the PNE content. Several exercises used in the exercise program will also be practiced in a functional way by the patient at home. Further details regarding the cognition-targeted exercise approach are described elsewhere.12
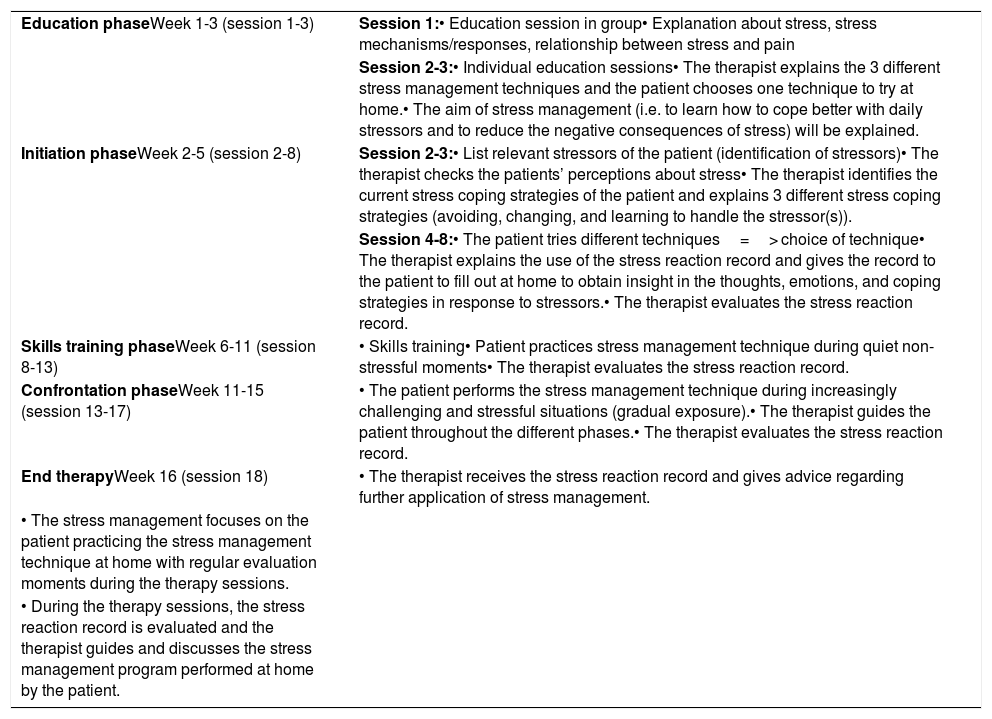
The experimental intervention will also include stress management (Table 3). The first step will consist of the education phase as described above. The therapist will ask during the individual education session whether the patient experiences stress and to what extent, and the stress coping strategies will be questioned. Subsequently, the therapist will explain different stress management techniques namely Jacobson's progressive relaxation therapy, Visualization, and Mindfulness. Next, the participant will choose one of these techniques to try at home in a quiet environment. Eventually the patient will choose one technique to perform the further stress management protocol.
Organization and content of the stress management program included in the experimental intervention.
| Education phaseWeek 1-3 (session 1-3) | Session 1:• Education session in group• Explanation about stress, stress mechanisms/responses, relationship between stress and pain |
| Session 2-3:• Individual education sessions• The therapist explains the 3 different stress management techniques and the patient chooses one technique to try at home.• The aim of stress management (i.e. to learn how to cope better with daily stressors and to reduce the negative consequences of stress) will be explained. | |
| Initiation phaseWeek 2-5 (session 2-8) | Session 2-3:• List relevant stressors of the patient (identification of stressors)• The therapist checks the patients’ perceptions about stress• The therapist identifies the current stress coping strategies of the patient and explains 3 different stress coping strategies (avoiding, changing, and learning to handle the stressor(s)). |
| Session 4-8:• The patient tries different techniques=> choice of technique• The therapist explains the use of the stress reaction record and gives the record to the patient to fill out at home to obtain insight in the thoughts, emotions, and coping strategies in response to stressors.• The therapist evaluates the stress reaction record. | |
| Skills training phaseWeek 6-11 (session 8-13) | • Skills training• Patient practices stress management technique during quiet non-stressful moments• The therapist evaluates the stress reaction record. |
| Confrontation phaseWeek 11-15 (session 13-17) | • The patient performs the stress management technique during increasingly challenging and stressful situations (gradual exposure).• The therapist guides the patient throughout the different phases.• The therapist evaluates the stress reaction record. |
| End therapyWeek 16 (session 18) | • The therapist receives the stress reaction record and gives advice regarding further application of stress management. |
| • The stress management focuses on the patient practicing the stress management technique at home with regular evaluation moments during the therapy sessions. | |
| • During the therapy sessions, the stress reaction record is evaluated and the therapist guides and discusses the stress management program performed at home by the patient. |
The next phase (i.e. initiation phase) consists of the try out session of the stress management technique(s) of the patient's choice at home and the identification of stressors that are relevant for the patient's life. Subsequently, the therapist will discuss patients’ perceptions about stress and explain various stress coping strategies. The patient will determine which coping strategy will be applied for each listed stressor. Afterwards, the participant will receive a stress reaction record to fill out at home. Next, the patient will practice the stress management technique during quiet moments during the skills training phase. Afterwards, the patient will perform the stress management technique during increasingly challenging and stressful situations (i.e. the confrontation phase).
Control intervention – biomedically focused education followed by exercise therapyPatients will participate in interactive educational sessions (biomedically focused neck school education) comprising 1 group session followed by 2 individual sessions. The education covers the normal course of neck pain, the anatomy, physiology, and biomechanics of the cervical spine, common mechanical causes of neck pain, the load-tolerance model, the importance of ergonomics, intradiscal pressure, and joint forces, lifting techniques, and the purpose and value of stretching and strength, endurance, and fitness training. Ergonomic principles like computer work and lifting techniques will be discussed. In addition, self-care training and the advice to remain active will be provided.
At the end of the first session, patients will be asked to first read an information leaflet and afterwards watch an online educational PowerPoint about the content of the neck school at home.
The neck school will prepare the patients for a symptom-contingent, biomedical approach to daily activity and exercise therapy. An individually supervised, patient-specific, graded, and active exercise therapy program will be performed during the next 13 weeks in 15 individual sessions. The symptom-contingent exercise program will focus on treating biomedical dysfunctions of the spine (e.g., mobility, strength) and general fitness exercises, with an evolution toward functional activities and physically demanding tasks while keeping the spine in physiologically neutral positions. Different therapeutic goals will be pursued depending on what emerges from clinical reasoning. The exercise program will consist of different types of exercises including warm-up, muscular endurance, strength, ergonomic, and flexibility exercises.
An activity form will be used. A graded progression of the ‘dosage’ of the symptom-contingent exercises will be emphasized. When the participant reports pain during or after an exercise, the intensity or duration of the exercise will be reduced. Several exercises used in the exercise program will also be practiced at home.
Treatment contrastThe main difference between the groups is the treatment of cognitive aspects of pain, stress management, targeting the brain, and accounting for central sensitization in the contemporary neuroscience group (biopsychosocial, time-contingent), which will not be applied in the control group (biomedical, symptom-contingent). Both interventions consist of an equal number of sessions and equal contact time with the therapist, and home exercises.
Primary and secondary outcome measures and assessment pointsOutcome measures will be assessed consistent with IMMPACT/OMERACT recommendations,33 and will be collected at baseline, after treatment completion, and at 6 months (primary endpoint and intermediate follow-up) and 12 months (long-term follow-up) after the end of treatment (Fig. 1). All assessors will be extensively trained.
Personal, demographic, and socio-economic characteristics will be collected at baseline. After treatment completion and at 6 months follow-up, all baseline tests will be performed again and all questionnaires will be completed. The 1 year follow-up will only include completing the questionnaires. Patient-reported outcomes will be completed online using LimeSurvey.
Primary outcome measureFunctional status was chosen as primary outcome measure. Individuals with chronic pain consider increased ability to function an important treatment objective.34 The Neck Disability Index (NDI) is the most commonly used self-report outcome measure for neck pain.35 The NDI will be used to investigate pain-related disability levels (0-50).36 The NDI is sensitive, valid, and reliable to assess self-reported disability.37,38
Secondary outcome measuresFor the evaluation of health-related quality of life, the 36-item Short-Form Health Survey Questionnaire (SF-36) will be used.39,40 The SF-36 has been shown to have good reliability and validity in patients with chronic pain.41
Current, average, and worst pain intensity will be assessed using an 11-point (0–10) numeric pain rating scale (NPRS). The NPRS has fair to moderate test-retest reliability in patients with neck pain.42 Also, pain frequency and its location will be assessed.
Central sensitizationThe central sensitization inventory (CSI) will be completed. This questionnaire measures self-reported symptoms related to central sensitization and its overlapping symptoms in people with chronic pain (0-100).43–46 The CSI has good internal consistency, good discriminative power, and excellent test-retest reliability.43
Endogenous pain modulation will be assessed by evaluating (1) electrical detection thresholds (EDTs) and electrical pain thresholds (EPTs),47 (2) endogenous pain facilitation,48,49 and (3) endogenous pain inhibition.49,50
Electrical detection and electrical pain thresholdsEDTs and EPTs will be measured unilaterally at the sural nerve of the leg and at the median nerve of both arms with a Digitimer DSA7 constant current stimulator (Digitimer, Hertfordshire, United Kingdom). The felt pad electrode for stimulation of the sural nerve will be placed 2cm posterior to the lateral malleolus, at the innervation area of the sural nerve,51 and will be placed on the skin overlying the median nerve for stimulation of the median nerve. The cathode of this electrode will be placed 5cm proximally from the wrist while the anodal electrode will be placed 3cm distally from the cathode.52 The order of test locations will be randomized. Each stimulus will be a constant current rectangular pulse train consisting of 5 pulses53 delivered at a frequency of 250Hz, each lasting 0.5ms54,55 (inter stimulus interval=3.5ms, total duration of 5 pulse train=20ms).
Stimulation will start at 0mA and will be gradually increased using steps of 0.5mA56,57 until the patient is experiencing a faint sensation (EDT) and until the stimulus is experienced as unpleasant (EPT).58 The mean EDT and EPT of 3 measurements with 30seconds in between will be used for further analyses. EPTs is a reliable measure.59
Endogenous pain facilitationSecondly, to examine endogenous pain facilitation, temporal summation will be assessed by delivering 20 electrical stimuli56 at the previously determined EPT intensity,58 with an inter-stimulus interval of 0.5sec. The participant will be asked to give a verbal numeric rating scale (VNRS) score ranging from 0 (no pain) to 10 (worst possible pain)56 after the 1st, the 10th, and the 20th stimulus. After this testing, there will be a rest period of 2minutes.
Endogenous pain inhibitionThirdly, the efficacy of endogenous pain inhibition will be assessed with the conditioned pain modulation (CPM) paradigm. The cold pressor task will be used as conditioning stimulus and electrical stimulation as test stimulus.60 Electrical stimulation will consist of 20 stimuli (250Hz, train of five), with a variable interstimulus interval of 8–12seconds, at 1.4 times EPT61 at the sural nerve and at the median nerve bilaterally.58 These stimuli will be given before (baseline condition) and during the application of the conditioning stimulus. Participants will be asked to immerse their hand up to the wrist in a bath (VersaCool, Thermo Fisher Scientific, USA) with circulating cold water maintained at 12°C for 3minutes (conditioned stimulus).58,62.
After applying the CPM paradigm at one body site the procedure will be performed at the second and third body site with a 3minute interval in between each test location.
For the baseline and CPM condition and for each test location, participants will be asked to score the overall experience of the electrical stimuli using a VNRS from 0 (no pain) to 10 (worst possible pain), after having received the 20 stimuli. CPM is a reliable measure with intersession reliability varying from fair to excellent.63
Quantitative electroencephalography recordings during rest and during the CPM paradigmQuantitative scalp electroencephalography (EEG) to examine brain activity will be recorded continuously using the eego sports system (ANT neuro, Enschede, The Netherlands) at a sampling rate of 2000Hz from 32 active surface Sn electrodes in a headcap with unipolar montage following the standard 10/20 recording system. The ground electrode is located in the active-shield cap fronto-centrally between the FPz and the Fz electrode. The reference electrode is located at CPz.
During the recordings, participants will be instructed to close their eyes, avoid blinking, and try to sit as still as possible. During the first 5minutes of the EEG recording, resting-state EEG activity will be measured. Afterwards, EEG will be collected during the assessment of CPM.
Self-reported psychological correlatesThe Pain Catastrophizing Scale, which has good psychometric quality, will be used to assess catastrophic thoughts and feelings about pain.64
The illness perception questionnaire-revised will be used to measure the illness perceptions of the patient. This questionnaire is reliable and has good construct validity and internal consistency.65
The Impact of Event Scale-Revised (IES-R) will be used to assess symptoms of posttraumatic stress.66 The IES-R is a self-report measure of current subjective distress in response to a specific traumatic event.67
The Pain Anxiety Symptoms Scale (PASS-20) will be used to evaluate pain-related fear, pain-related anxiety responses, and fear-avoidance behavior.68,69 The PASS-20 has good predictive and construct validity and strong internal consistency and reliability.69
Socio-economic outcomesHealth care use and health-related costs will be evaluated using the combination of 2 self-reported valid questionnaires: (1) the Medical Consumption Questionnaire,70 a generic instrument for measuring direct medical costs of a patients’ total medical consumption, and (2) the Productivity Cost Questionnaire71 to obtain data regarding the indirect costs outside health care, but related to the disease.72
BlindingParticipants will not know if they receive the experimental or control intervention, however, they will be aware of the intervention received. Additionally, outcome assessors and the statistician will be blind to the treatment groups for all primary and secondary outcomes. After each post-treatment assessment moment, the success of assessor blinding will be examined by asking whether the assessor thinks the patient received the experimental or control intervention, including the percentage of certainty. The same method will be used to assess the success of patient blinding for treatment group. The therapists providing the experimental intervention will not be involved in providing the control intervention, and vice versa.
Additional protocol items following the SPIRIT statementInformation concerning adherence, concomitant care, and interventions, retention, statistical analyses, ethics, the data security and management plan, and monitoring can be found in Table 4.
Additional protocol items following the standard protocol items for randomized interventional trials (SPIRIT statement).
| Adherence |
| The degree of treatment adherence will be measured with the amount and frequency of therapy sessions (out of 18) completed by the participants in combination with performance of the home exercises. Treatment adherence will be measured by discussing with the patients during prespecified treatment sessions which and how many home exercises they performed. The patients will also provide this information via a home exercise diary that is used to assess therapy compliance. Additionally, the therapist will ask during the first individual education session whether the patient has read the online PowerPoint education and the educational information leaflet at home. Detailed information from each participant about number, dates, and frequency of completed sessions, and if applicable reason and moment of dropout or loss to follow-up will be recorded. |
| Concomitant care and interventions |
| Participants in both groups will be allowed to take their usual medication but not to follow other concomitant physical and psychological therapy interventions during the 16 weeks of treatment. Treatments received outside of the trial treatment will be recorded during the treatment and follow-up phase. If surgery needs to take place during the treatment period, the participant will be excluded, and this will be documented. |
| Data collection method: retention |
| To promote participant retention and completion of follow-up assessments, the assessor will contact the participants prior to the assessments, reminding them of the upcoming data collection and the incentive they will receive when completing the assessments. Specifically, participants will receive a gift voucher at the 6 months follow-up assessments and a second gift voucher at the 1 year follow-up assessment when completing all assessments of the respective test moment. If the participant is willing to complete the online questionnaires at home but is not willing to complete the laboratory-based experimental measurements at the 6 months follow-up and/or the test moment immediately after the treatment, the participant is allowed to only complete the questionnaires. However, completing all assessments is encouraged, and the reasons for not completing assessments are noted. Additionally, at the end of each therapy session the next therapy session is already scheduled, and the laboratory-based assessments will be scheduled sufficiently ahead of time. |
| Statistical analyses |
| Data analysis will be conducted based on intention to treat principles. Intention to treat analysis is generally favored because it avoids bias associated with non-random loss of participants and corresponds to analyzing the groups exactly as randomized regardless of whether they received the randomized treatment.77 To avoid selection bias, we will perform an ‘as randomized’ analysis which retains participants in the group to which they were originally allocated. To prevent attrition bias, outcome data obtained from all participants will be included in the data analysis. These two conditions (i.e., all participants, as randomized) define our intention to treat analysis, which is recommended as the preferred analysis strategy.77 We will consult the Biostatistics Unit of Ghent University when performing these analyses. We will record and report reasons for missing data for each randomization group. Additionally, pre-specified subgroup analyses will be performed. Factors associated with poor outcome and factors associated with clinically important changes in the outcome measures following treatment will be assessed. The trial is not specifically powered for these subgroup analyses. The study is powered for the primary outcome parameter ‘pain-related disability’ at 6 months follow-up. Interim analyses have not been performed. |
| Ethics |
| Approval to conduct this study was granted by the ethics committee of the University Hospital Brussels (2016/388), Ghent University Hospital (2017/0850), and Sint-Jozefkliniek Campus Bornem (1811CP175). This study was registered with the ClinicalTrials.gov Identifier No. NCT03239938. The trial will be conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. Participants will give their written informed consent prior to participation in the study. |
| Monitoring |
| The study will monitor adverse events during the treatment (e.g. hospitalization) and by asking questions to the patients including questions about changes in medication use immediately after the end of the treatment. Furthermore, at 6 and 12 months follow-up, questions concerning medication use, hospitalization, emergency visits, overnight stays (e.g. in rehabilitation centers), and consultations with medical specialists and healthcare providers for physical and/or psychological complaints will be asked. |
| Data security and management plan |
| Participant data are stored on a secure database in accordance with the General Data Protection Regulations (2018). All personal identifiable information and clinical trial data will be separated. Data are de-identified and a unique trial identification number will be used, and the link between personal identifiable data and this identification number will be stored securely and separately from trial data (pseudonymisation). All personal information collected from the patients will be stored and analyzed confidentially. Electronic records of the data will be generated and saved to the University servers which are automatically backed up daily. Responses of the online questionnaires completed using LimeSurvey will be saved on a secure password protected University server which will only be accessible by the researchers. |
| The responsible person to preserve the trial data during and at least 10 years after completion of the trial will be the project coordinator (IC), who will be supervised by the principal investigator (JN). In case the project coordinator does not continue working at the Vrije Universiteit Brussel or Ghent University in the 10 years after completion of the trial, the principal investigator will take over the role as responsible person. Along with the coordinator and the principal investigator, project team members will have responsibility for study-wide data management, data quality assurance, and security. |
Baseline data will be analyzed to determine descriptive statistics for the outcome measures. Analyses will be performed to unravel relationships between functional status, pain, psychological correlates, and central sensitization. A linear mixed models analysis will be used to evaluate and compare therapy effects. Data analysis will be conducted following intention to treat principles. Effect sizes, 95% confidence intervals, and statistically and clinically significant differences will be calculated. We will consider a between-group difference of 3.5 points on disability (NDI) and 1 point on pain intensity (NPRS) to be minimal clinically important.73,74
DiscussionThis study will provide novel data on the effectiveness of a contemporary neuroscience approach compared to biomedically focused education followed by symptom-contingent exercise therapy on key patient-centered outcome measures and various measures of central sensitization in people with chronic WAD.
Important study strengths include the multi-center study design, blinded outcome assessments up to 1-year follow-up, the validated and reliable outcome measures, the randomized controlled study design, and the balanced treatment arms. This trial may provide new and improved treatment guidelines for health care professionals for the treatment of people with chronic WAD, and may be used as a basis for recommendations to health authorities.
Conflict of interestThe authors have no conflicts of interest. Jo Nijs has co-authored a Dutch book for clinicians on pain neuroscience education and has authored a Dutch book for clinicians on central sensitization in clinical practice, but the royalties for those books are collected by the Vrije Universiteit Brussel and not him personally.
The authors gratefully acknowledge Roos Colman from the Biostatistics Unit of Ghent University for the preparation of the randomization lists. This work was supported by the Research Foundation Flanders (FWO), Belgium [grant number G007217N]. The funder will have no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, approval, or submission of any manuscript that will emerge from this study.